To understand this section you should have a solid grounding in calculating the pH or pOH of weak acid or base solutions (see here).
pH Buffering — the idea
Many chemical systems can only do what you'd like them to do within strict limits of pH, so we need a way to control pH and make a solution resistant to changes in pH.
Biological systems are particularly sensitive to fluctuations in pH and are always buffered (protected) from those changes somehow. When we do biological experiments on the bench, we have to carefully design solutions so that they're buffered against pH changes.
For example, the pH of your blood is maintained by a chemical system within it to remain between 7.35 and 7.45 pH units. That's narrow. A pH below 7.35 makes you acidotic and you will need medical intervention soon. The opposite of acidosis is alkalosis, where blood pH rises above 7.45, and it's equally dangerous for you.
In this section we'll figure out how this buffering against pH change is done. It's very elegant and worth understanding. I think it's pretty cool.
pH Buffers
pH Buffers are solutions that resist changes in pH. They aren't perfect, and each buffer system works over a range of a couple of pH points or less.
First: Conjugate acids and Conjugate bases
Before we get to buffers we need to define a conjugate acid and a conjugate base. Here's the dissociation of the strong acid H2SO4 (we'll just lose one of the acidic protons here):

H2SO4 is a strong acid (one you've memorized), and it's conjugate base is a weak base. In general that pattern holds for all acids and bases, so we'll repeat it in the box below. Note that because reaction arrows can point either way, H2SO4 is also the conjugate acid of the base HSO4-
Strong acids and bases dissociate completely in water.
Strong acids
| HCl | hydrochloric acid |
| HBr | hydrobromic acid |
| HI | hydroiodic acid |
| HNO3 | nitric acid |
| HClO3 | chloric acid |
| HClO4 | perchloric acid |
| H2SO4 | sulfuric acid |
Strong bases
| LiOH | lithium hydroxide |
| NaOH | sodium hydroxide |
| KOH | potassium hydroxide |
| RbOH | rubidium hydroxide |
| CsOH | cesium hydroxide |
| Ca(OH)2 | calcium hydroxide |
| Sr(OH)2 | strontium hydroxide |
| Ba(OH)2 | barium hydroxide |
Conjugate acids & bases
- The conjugate base of a strong acid is a weak base.
- The conjugate base of a weak acid is a strong base.
- The conjugate acid of a strong base is a weak acid.
- The conjugate acid of a weak base is a strong acid.
Here's the dissociation of a weak acid, acetic acid, CH3COOH. I've drawn the reaction arrows with different lengths to show that the reaction equilibrium lies mostly to the left. That is, most of the acetic acid remains undissociated because it's weak. Another way to view it is that the acetate ion, CH3COO- is a strong base that readily grabs up any protons it encounters (that's what bases do) to push the equilibrium back toward acetic acid.

Yet another way to view this equilibrium is that the rate of the forward reaction is slower than the rate of the backward reaction. We'll get to that point of view when we study chemical kinetics later.
Finally, here's the reaction of the base ammonia (NH3) in water to give a conjugate acid, NH4+.

The ammonia reaction is a good one to look at because it reminds us that water can act either as acid or base. Water follows the same rules: H2O is a weak acid and its conjugate base, the hydroxyl ion, is a strong base.
OK, with that out of the way, let's make some buffer systems and see how they work ...
Buffers
A buffer system consists of either
A weak acid and a salt of its conjugate base or
A weak base and a salt of its conjugate acid.
Example 1
The Acetic acid – sodium acetate buffer system
This buffer system will consist of the weak acid acetic acid, CH3COOH, and the salt of its conjugate base, sodium acetate, CH3COONa.
The acid dissociation and its Ka are:

The dissociation of this soluble salt looks like this:

Notice that we can add all of the sodium acetate we want to this solution without changing the pH. It does change a (very) little due to Le'Chatelier's principle; excess acetate pushes the acid equilibrium to the left, but not too much because not much of the acid is dissolved anyway.
What happens when we add acid to this solution?
When we add acid (we'll assume it's strong acid), the additional protons (H+) will combine with abundant acetate ions to form acetic acid. The small Ka of acetic acid ensures that most of this doesn't ionize, thus the pH change will be very small. The solution has basically soaked up and masked or hidden the added H+ ions.
What happens when we add base?
When a source of OH- is added to this solution, protons from acetic acid bind to the OH- to form neutral water. This causes a shift in the acid equilibrium to the right, as more acetic acid is ionized to re-establish equilibrium. Remember that because of the low Ka of acetic acid we have a large "reservoir" of undissociated CH3COOH.
Basically, a buffer solution consists of a reservoir of acid that will neutralize base and a reservoir of base that will neutralize acid. Each is present in large amounts and available to be used as needed.
The panel below shows the buffering mechanism of an acidic buffer in a cartoon. Understanding the diagram in all its details will help you to understand how buffers work.
Mechanism of acidic buffer action

Limitations
Of course, we could always dump in so much acid or base that we completely swamp the concentrations of our buffer components, so we've got to make sure we have a high-enough concentration of buffer for our needs. Typically for biological applications, buffer concentrations are on the order of 5 to 100 millimolar (0.005 to 0.1M). We dont' want the buffer components themselves to become players in whatever reaction we're studying, so there has to be some compromise in our design, usually by trial and error.
Even then, buffers only really buffer the pH in the manner described within a pH point or so of the pKa of the weak acid or the pKb of the weak base.
What's with CH3COOH ?

Why is the formula for acetic acid written as CH3COOH rather than C2H4O2 ?
Aceitic acid is one of many organic acids called carboxylic acids. The carboxylic acid group, which all carboxylic acids share, looks like this:

where R stands for any number of organic compounds that could be attached to the carboxyl group, forming a carboxylic acid. In the case of acetic acid, R = CH3
Writing the formula of a carboxylic acid as RCOOH is a way of making note of the structure of the molecule and its identity as a weak acid.
Buffers resist pH change ... but only to a point
This graph shows the pH of pure water and two acetic acid buffer solutions (0.1M acetate and 0.2M acetate) as the concentration of added HCl is increased from 0 M to 0.1 M.
It's no surprise that the pH of the unbuffered water changes linearly with the amount of added acid (black line).
The buffered solutions maintain pH 4.76 (the pKa of the CH3COOH) over about four pH points. That's just the kind of behavior we want from a buffered solution: Add acid and the pH doesn't change.
The more concentrated buffer (blue curve) is affected less than the less concentrated (0.1M) buffer.

Working range of a buffer (approximate rule)
Buffers work best within about ± 1 pH point of the pKa of the weak acid or pKb of the weak base of the buffer system.
Example 2
pH after addition of some strong acid
Now let's actually calculate the pH change when we add some HCl to 500 ml of an acetate buffer system. Let's assume a 50 mM (0.05 M) buffer concentration consisting of 0.025M CH3COOH and 0.025M CH3COONa, and let's add 1 ml of 6 M HCl.
$$HCl \longrightarrow H^+ + Cl^-$$
$$ \begin{align} 1 \, ml \, HCl &\left( \frac{1 \, L}{1000 \, mL} \right) \left( \frac{6 \, mol}{1 \, L} \right) \\[5pt] &= 0.006 \; mol \; H^+ \end{align}$$
Now we note that we only have half a liter of our buffer so we have 0.025 moles each of acetic acid and sodium acetate. It's important to have everything in terms of moles so that we get the stoichiometry right.
Next we make a table (an "ICE" table) to get all of our facts straight. Because acetic acid is a weak acid, we assume that it ionizes very little. On the other hand, sodium acetate is a strong electrolyte (is very soluble), so we have as many moles of acetate ion as we began with CH3COONa. Any acid introduced will bind to acetate ions, and most of that will remain as undissociated CH3COOH.
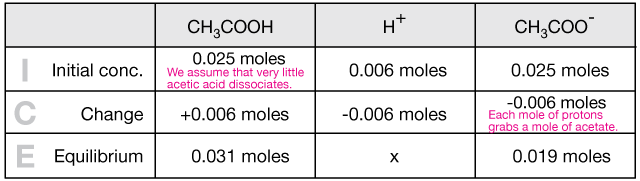
Now we take the equilibrium expression,

and rearrange it to solve for the concentration of protons. The negative base-ten log gives us the pH of the solution after addition of 0.006 moles of HCl:

The pH of the buffer before addition of the acid was 4.76, so adding quite a bit of a strong, concentrated acid only changed the pH by a little.
Example 3
pH after addition of some strong base
Let's finish up with our acetate buffer by adding some strong base (a new buffer, not the one above with all the acid in it). Let's calculate the concentration of 500 ml of a buffer solution consisting of 0.025M CH3COOH and 0.025M CH3COONa after adding 0.5 g of solid NaOH.
$$0.5 \, g \, NaOH \left( \frac{1 \, mol}{40 \, g \, NaOH} \right) = 0.0125 \, mol \, OH^-$$
Then, using the same numbers of moles of acetic acid and acetate that we calculated in the last example, we set up this table:

Now we calculate the concentration of protons and the pH in the same manner as the last example ... it's plug and chug:

That's twice the number of moles of base than acid from the prior example, but the pH still didn't change by all that much: 4.76 to 5.25, or half of a pH point.
pH of a buffer solution:
The Henderson-Hasselbalch equation
Let's see if we can calculate the pH of our buffer solution. We'll do it with a "generic" weak acid, HA, where A- is the anion. The dissociation equilibrium is:
$$HA \rightleftharpoons H^+ + A^-$$
The Ka expression is then just:
$$K_a = \frac{[H^+][A^-]}{[HA]}$$
Now let's calculate the pKa of this acid by taking the negative base-ten log of both sides.
$$=log_{10}K_a = -log_{10} \left( \frac{[H^+][A^-]}{[HA]} \right)$$
Now using the laws of logs (namely, the log of a product is the sum of logs), we can separate some terms like this:
$$=log(K_a) = -log[H^+] - log\left( \frac{[A^-]}{[HA]} \right)$$
Now we just recognize the pH hidden in there, and rearrange to get the Henderson-Hasselbalch equation for the pH of a buffer.:
$$pH = pK_a + log\left( \frac{[A^-]}{[HA]} \right)$$
In this case the [A- ] term contains contributions from both the weak acid and its salt.
The Henderson-Hasselbalch equation
The Henderson-Hasselbalch equation is the key formula to use for calculating pH of a buffer system.
$$pH = pK_a + log\left( \frac{[A^-]}{[HA]} \right)$$
Application to the carbonate buffer system
The carbonate buffer system is a key component of the blood pH buffering system. Carbon dioxide, a biproduct of cellular respiration, dissolves in the blood to form this buffer.

In the blood, CO2 combines with water in the double equilibrium,
$$CO_2+H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons HCO_3^- + H^+$$
The relevant carbonic acid equilibrium for this example is
$$H_2CO_3^- + H_2O \leftrightarrow HCO_3^{2-} + H_3O^+$$
with Ka =4.3 × 10-7 and pKa = 6.36
One way to make a carbonate buffer of a known concentration is to keep on hand stocks of NaHCO3 and Na2CO3 of a known concentration, then mix them proportionally and dilute them to the desired concentration.
Let's alculate the pH of a solution formed by mixing 4 ml of 0.2M Na2CO3 with 46 ml of 0.2M NaHCO3.
$$pH = pK_a + log \left( \frac{[HCO_3^-]}{[H_2CO_3]} \right)$$
Now we calculate the number of moles of each ion in the expression. We derive HCO3- ions from the soluble NaHCO3 salt, so we have
$$ \begin{align} \require{cancel} 46 \; ml \; NaHCO_3 &\left( \frac{\cancel{1 \, L}}{1000 \, mL} \right) \left( \frac{0.2 \, mol}{\cancel{1 \, L}} \right) \\ [5pt] &= 0.0092 \; mol \end{align}$$
We also need the number of moles of carbonic acid. The source of that is Na2CO3, which dissociates in water completely, leaving
All-in-one buffers
Sometimes, and particularly in biology, we choose to use single-component buffers, buffers that act both as an acid and a base to fend off acid or base challenges to the pH of a solution. This buffer, TRIS for short, has a pKa of 8.07, so it's a good buffer to keep a solution between pH 7 and pH 9.
TRIS is dissolved in solution with a little acid so that both the acidic and basic forms are around.
TRIS might be the most frequently-used buffer in biological experiments.

HEPES: A Zwitterionic buffer
The HEPES (pronounced like heaps, and stands for 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid)) buffer is a Zwitterionic buffer.
A Zwitterion can carry both a positive and negative charge at once. That's what allows HEPES to have both a weakly acidic part and a weakly basic part in solution. Recall that all of the free amino acids are zwitterions.
HEPES has a pKa of 7.5. It is considered to be a good buffer to keep soutions between a pH of 6.8 and 8.2, and is the most frequenly-used buffer in the culture of mammalian cells.

Practice problems
Here are some sample buffer calculation problems. You can download handwritten solutions to these using the button below.
-
Calculate the pH of a buffer formed from 50 ml of 15.0 M NH3 and 53.5 g of NH4Cl dissolved in enough water to make 500 ml of solution.
Answer
The relevant equations are:
NH3 + H2O ↔ NH4+ + OH-
Weak base NH4Cl → NH4+ + Cl-
salt Initial concentrations:
NH3
$$\frac{15 \, mol}{1 \, L} (0.05 \,L) = 0.75 \; mol \; NH_3$$
NH4Cl
$$ \begin{align} 53.5 \, g \, NH_4Cl &\left( \frac{1 \, mol}{53.5 \, g \, NH_4Cl} \right) \\[5pt] &= 1 \; mol \, NH_4^+ \end{align}$$
Now let's derive the HH equation and use it to find the pH:
$$ \begin{align} K_b &= \frac{[NH_4^+][OH^-]}{[NH_3]} \\[5pt] log(K_b) &= log\left( \frac{[NH_4^+]}{[NH_3]} \right) + log[OH^-] \\[5pt] pK_b &= log \left( \frac{[NH_4^+]}{[NH_3]} \right) - pOH \\[5pt] pOH &= pK_b + log \left( \frac{[NH_4^+]}{[NH_3]} \right) \\[5pt] &= 4.75 + log \left( \frac{1/0.5 \, L}{0.75/0.5 \, L} \right) \end{align}$$
$$pOH = 4.87 \; \longrightarrow \; \bf pH = 9.12$$
-
Calculate the pH of the previous solution after addition of 100 ml of 0.2 M NaOH. Remember to calculate the number of moles of OH-, then calculate a new [OH-] using the new volume.
Answer
The relevant equations are:
NH3 + H2O ↔ NH4+ + OH-
Weak base NH4Cl → NH4+ + Cl-
salt NaOH is a strong base, so it dissociates completely. The amount of OH- introduced to the solution is
$$0.1 \, L \left( \frac{0.2 \, mol}{1 \, L} \right) = 0.02 \, mol \; OH^-$$
-
Calculate the pH of a solution made by adding 0.04 g of NaOH (FW = 40.0 g/mol) to 100 ml of a 0.4 M solution of CH3COOH (acetic acid). Is it a buffering solution (i.e. is the pH of the solution close to the pKa of acetic acid?
Answer
Sodium hydroxide (NaOH) is a strong base, so it dissociates completely in water. A 0.04g sample of NaOH contains
$$(0.04 \, g \, NaOH)\frac{1 \, mol}{40 \, g} = 0.001 \, moles \, OH^-$$
Now the number of moles of the weak acid is
$$\frac{0.4 \, mol}{1 \, L} (0.1 \, L) = 0.04 \, mol \, CH_3COOH$$
The 0.001 moles of OH- will neutralize 0.001 mol of acetic acid, leaving 0.039 mol of acetic acid plus 0.001 mol of NaCOOH, the sodium salt of the weak acid. From here we can do the calculation in two ways.
Method 1: Find the pH of a weak acid solution using the Ka of the acid. First make an ICE table:
$$ \begin{array}{llrr} component & I & C & E \\[3pt] CH_3COOH & 0.039 & -x & 0.039 - x \\[3pt] CH_3COO^- & 0 & +x & x \\[3pt] H^+ & 0 & +x & x \end{array}$$
Now for acetic acid, $K_a = 1.738 \times 10^{-5},$ so our equilibrium expression is
$$1.738 \times 10^{-5} = \frac{[x][x]}{0.039 - x}$$
Because Ka is small, we can make the approximation $0.039 - x \approx 0.039,$ and solve
$$ \begin{align} x^2 &= 0.039 (1.738 \times 10^{-5})\\[5pt] &= 6.7782 \times 10^{-7} \\[5pt] x &= 8.23 \times 10^{-4} = [H^+] \end{align}$$
So the pH is 3.08
Method 2: Use the Henderson-Hasselbalch equation:
$$ \begin{align} pH &= pK_a + log \left( \frac{[A^-]}{[HA]} \right) \\[5pt] &= 4.76 + log \left( \frac{0.001/0.1}{0.039/0.1} \right) \\[5pt] &= 4.76 - 1.59 = 3.17 \end{align}$$
-
Calculate the pH of a buffer that is 0.12M in lactic acid (“HL”, pKa = 3.9) and 0.11M in sodium lactate (“NaL”).
Answer
Solution to follow: Stand by
-
Calculate the pH of a buffer formed by mixing 85mL of 0.13M lactic acid (pKa = 3.9) with 95mL of 0.15M sodium lactate.
Answer
Solution to follow: Stand by
-
Calculate the percent ionization of 0.050M butanoic acid (“HB”, Ka = 1.5 x 10-5).
Answer
Solution to follow: Stand by
-
Calculate the percent ionization of 0.050M butanoic acid in a solution containing 0.070M sodium butanoate (“NaB”).
Answer
Solution to follow: Stand by
-
A buffer solution contains 0.10 mol of acetic acid and 0.13mol of sodium acetate in a total volume of 1.0 liters.
- Calculate the pH of this buffer.
- Calculate the pH of the buffer after addition of 0.02 moles of KOH.
- Calculate the pH of a liter of pure water after addition of 0.02 moles of KOH.
- Calculate the pH of the buffer (in part a) after addition of 0.02 moles of HNO3.
- Calculate the pH of a liter of pure water after addition of 0.02 moles of HNO3.
- Calculate the pH of a liter of 0.01 M HCl solution after addition of 0.02 moles of KOH.
Answer
Solution to follow: Stand by
The flu: An interesting story of pH
Here's an interesting story of how a change in pH can lead to profound chemical change.
The influenza (flu) virus has an amazing way of entering your cells (see the image below). It begins with the immune system tagging the virus as an intruder, "marking" it for a process called endocytosis, in which the virus is pulled inside a healthy cell inside an endosome, a sphere of membrane formed from the fatty cell membrane. Endocytosis keeps the virus isolated from the cell innards.
There are two key proteins on the surface of the flu virus, hemagglutinin (H) and neuraminidase (N). You might have heard of flu strains like H1N1 and H5N1; those are ratios of H to N that characterize that strain.
At normal cell pH, around 7.4, the hemagglutinin folds up in such a way as to bury what is called its fusion peptide,
a short piece of protein capable of pushing through a cell membrane. One thing that occurs during the process of endocytosis is that the pH of inside the endosome drops to 5. At this pH, hemagglutinin rearranges dramatically to "unsheathe" this peptide, allowing it (and two others arranged in a triad or "trimer") to poke through the endosome membrane, fuse it with the viral membrane and thus open up a channel through which the viral RNA can enter the cell and make copies of itself. The cell is then infected.
The rearrangement is shown in the ribbon diagram of hemagglutinin on the right side of the figure below. You can learn a little more about proteins here.
It's a remarkable adaptation of the virus to this pH change in the cells of its mammalian hosts.
Entry of influenza virus into a cell


![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.