Some substances dissolve more easily than others.
In the photo on the right, a solution of lead nitrate, $Pb[NO_3]_2$, is being added to a solution of potassium iodide $(KI)$. The double displacement reaction that occurs is:
$$2KI_{(aq)}+Pb(NO_3)_{2 \; (aq)} \rightleftharpoons 2KNO_{3 \; (aq)} + PbI_{2 \; (s)}$$
Potassium iodide and lead nitrate are soluble salts. That means when they are added to water, nearly all of the salt dissolves in the solvent (water), forming the clear solutions initially present in the beaker and the graduated cylinder.
When the two are mixed, the potassium nitrate that is formed is another soluble salt, and remains fully dissolved. The yellow lead iodide (PbI) that is formed is insoluble; very little of any quantity of PbI dissolves in water. Instead it forms a yellow solid that falls to the bottom of the beaker—a precipitate.
In this section we'll try to do two things. First, we'll set out some rules, the solubility rules, and (sorry) exceptions to those that can help us tell at a glance whether some substance might dissolve in water, then we'll develop a mathematical model of solubility called the solubility product. It will allow us to get quantitative about how much of a given ionic compound dissolves in water.

Terminology
In a solution we dissolve a solute — the thing being dissolved — into a solvent. In this section we'll be mainly using water as the solvent (aqueous solutions).
Not all substances dissolve as easily as the next in a given solvent. For example, we can dissolve over 350 g of $NaCl$ in a liter of water, but less than 2g of $Ca(OH)_2$ will dissolve in that same volume. Some substances (solutes) are more soluble than others in a given solvent, that is, more can dissolve in the same amount of solvent. A precipitate is solid matter that does not or can no longer be dissolved in the solution.
Sometimes chemists say that a salt "crashes out" of solution when it precipitates. That's probably because precipitation can sometimes be dramatic, with solid material materializing almost instantly and falling to the bottom of a beaker.
For example, if the salt AB is dumped into water, it splits into ions: $AB \rightleftharpoons A^+ + B^-$. If $AB$ is only partially soluble, then some $AB$ will not dissolve, and usually ends up sinking to the bottom of the container.
When a solution is holding all of the solute it can, and some of the solute has either precipitated or is about to, we call it a saturated solution

In a saturated solution, it doesn't matter how much of the solid is present, just that there is some. If there is a precipitate, the solution is saturated.
What it means to dissolve
When an ionic compound (a salt) dissolves in water to form an aqueous solution, its cation(s) and anion(s) break apart and are surrounded by water. Recall that the water molecule is bent (see VSEPR theory) because of the presence of two lone pairs of non-bonding electrons. This gives water a slightly positive (δ+) and a slightly negative (δ-) "end." That is, water is polar.
When a cation such as sodium (Na+, say from dissolving sodium chloride in water) is released from its ionic bond in the solvent, it is surrounded by water molecules that orient their negative ends toward the positive ion.

The figure shows one of the waters with its lone pairs represented. These waters aren't fixed in place in these orientations; instead, they are translating and rotating in the solution almost as usual in pure water. They do, however, spend more of their time in orientations something like those illustrated.
Likewise, when an anion like the chloride (Cl-) ion is placed in water, it tends to be surrounded by waters with their positive ends oriented toward it.

Again, the effect is transient. Each water is free to rotate and move around in solution, but on average, a chloride ion "sees" mostly the positive ends of the water molecules surrounding it.
We call this first layer of water molecules (and remember that it's a 3-dimensional sphere) the first solvation shell around the ion. There can be a second shell and a third, which form because of the preferential orientation of the waters of the previous shell, though the effect wears off after a few shells. The tendency of water molecules to orient is referred to an anisotropy. To be isotropic is to be insensitive to position, especially rotation. In solution, water molecules are mostly isotropic, but the introduction of a charged solute introduces an an-isotropy – a lack of isotropy.

The illustration above shows what a 2-D slice of the first three aqueous solvation shells surrounding a cation might look like. Solvation shells become more transient and less organized (thus more blurry in the figure) as we move farther out from the ion, whether the solute is a cation or an anion.
There are patterns in the solubility of compounds, and those have been written down — the solubility rules. The are general rules for predicting the relative solubilities of various ionic compounds—salts—in water. These need not be memorized, but a discussion of them might help you understand why some substances are more soluble than others.
I hope that with experience, you'll remember many of these rules without rote memorization. They do come in handy when you need to make quick decisions about an experiment.
I know that it will seem like there are a lot of exceptions to these rules, but I assure you that each is explained when we look at the underlying electronic structure of the ions involved.
Compounds like LiCl, NaNO3, KOH, and RbF are soluble. That means when these species are present in an aqueous solution almost none of the "parent" compound is present. It's all dissociated into its constituent ions. For example, when NaCl is added to water, the dissociation reaction
$$NaCl \longrightarrow Na^+ + Cl^-$$
goes to completion and only the ions will be detectable in the solution. This rule holds only, of course, if the concentrations of the salt isn't extremely high. You can dissolve over 350 g of NaCl in a liter of water. That's a lot, but there is a limit. If you try to dissolve much more, it won't go into solutions and will just fall to the bottom of the beaker as solid NaCl.

Notice, as you work through the solubility rules below, that for a number of fairly insoluble anions, coupling of these with an alkali metal will 'rescue' the solubility.

All ammonium salts like NH4OH, NH4Cl and (NH4)2SO4 are completely soluble in water.
Dissolved ammonium compounds give off the pungent smell of ammonia. The ammonia-based cleaners in your house are usually aqueous solutions of ammonium hydroxide, NH4OH.
A salt that evaporates
Ammonium acetate, composed of the ions NH4+ and CH3COO-, is sometimes used in solutions in which one would like the salt to evaporate over time. Both the ammonium and acetate ions are volatile and will evaporate. Note that salts like HCOONa or NH4Cl will not evaporate because the counter ions Na+ and Cl- would be left behind, leaving a charge imbalance.
All salts containing these molecular anions are soluble. They include compounds like
| NH4CH3COOH → NH4+ + CH3COO- | ammonium acetate |
| NaNO3 → Na+ + NO3- | sodium nitrate |
| KClO3 → K+ + ClO3- | potassium chlorate |
| LiClO4 → Li+ + ClO4- | lithium perchlorate |
Notice that you can combine some of the solubility rules here. Ammonium acetate contains the ammonium ion (see rule 2) and the last three in the list contain alkali metal salts (see rule 1). It's helpful to look for patterns like that. It makes this list significantly less daunting.
By the way, you've probably seen that prefix "per" a few times by now. Here's what it means:

Most salts containing Cl-, Br- and I- are soluble, however there are exceptions. PbCl2, HgCl2 and AgCl, for example, are not soluble.
The photo on the right shows what happens when soluble AgNO3 is added to a solution of soluble NaCl. The resulting double displacement reaction is:
$$AgNO_{3 \;(aq)}+NaCl_{(aq)} \rightleftharpoons NaNO_{3 \; (aq)} + AgCl_{(s)}$$
The insoluble AgCl precipitates from the solution as a solid. Such a reaction can be a nice quick, visual way of determining whether a solution contains any soluble Ag+ ions—just precipitate them by adding some chloride and look for the cloudy white precipitate.

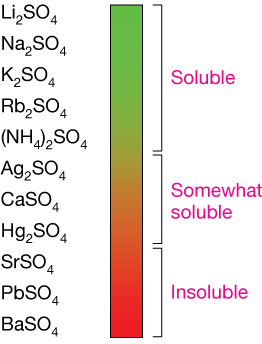
The sulfate ion (SO42-) is very soluble, but some cations that pair with it bind so tightly that they don't ionize easily in water.
Sulfates of the alkali metals are completely soluble. Sulfates of silver (Ag), calcium (Ca) and mercury (Hg) are somewhat soluble. A solution will hold just a little dissolved CaSO4 before any additional CaSO4 precipitates.
The other sulfates shown are insoluble. Only the tiniest amounts are ionized when solid material is added to water.
Other hydroxide compounds, such as Fe(OH)2, Mg(OH)2 and AgOH are insoluble. Halide hydroxides like NaOH, LiOH and KOH completely dissociate in water.
Ba(OH)2 dissociates enough to make it a strong base, a corrosive substance that adds free OH- ions to solution.
When you encounter a hydroxide compound that doesn't contain an alkali metal cation, a red flag should go up — this might not be very soluble.


All other sulfide compounds are ridiculously insoluble.
The compounds listed here are roughly a factor of 10 to 100 less soluble than the one above. Sulfides aren't very soluble in general. Be on the lookout for insoluble sulfides. They stink and they can make a mess of your glassware.
All other salts that form with these anions are insoluble.
Should I memorize the solubility rules?

You should follow your teacher's advice, but I think with a little practice using these rules you'll develop an intuition about which ionic compounds are soluble and which set off "red flags" in your mind. Knowing the first two solubility rules will get you a long way, and having a general wariness about sulfides, hydroxides and salts of the heavier metals will help. You should remember that the rules exist and know where to find them if you need them (your phone is a good place). The next section should help, too.

Kidney stones — a (painful) lesson in solubility
Kidney stones are just precipitates, usually of the insoluble salts calcium oxalate or calcium phosphate. A small bit of precipitate becomes the nucleus for a larger crystal like the ones shown here. The larger one is about 8mm across. They form when there is too much calcium in the bloodstream and the kidneys are not flushed with enough water.
Well, the solubility rules are nice, but they're kind of inexact. In science we always strive to be more quantitative. We don't just want to know that Fe(OH)2 is more soluble in water than Fe(OH)3, we want to know how much more soluble it is.
We solve this problem by inventing the solubility product, $K_{sp}$ (sp = "solubility product"), a constant unique to every ionic compound, and which can be measured and used in calculations.
We will define the $K_{sp}$ in more detail in the section on chemical equilibrium, but for now, simply accept the definition here. We start with a generic dissociation reaction:
$$AB \rightleftharpoons A^+ + B^-$$
In this reaction the ionic compound $AB$ dissociates into the cation $A^+$ and the anion $B^-$. Recall that we abbreviate molar concentration with square brackets: $[A^+]$ means "the molar concentration of $A^+$."
We now "invent" the solubility product expression as the product of the molar concentration of the dissociated ions:
$$A_mB_n \rightleftharpoons mA^+ + nB^-$$
It turns out that this product will be constant for a given ionic compound once equilibrium is established between the solid and the ions. We need to go one step further and account for compounds that can break into multiple copies of the same ion, like Fe(OH)3. To do that, we change our generic reaction to
$$A_mB_n \rightleftharpoons mA^+ + nB^-$$
and write the $K_{sp}$ equation as
$$K_{sp} = [A^+]^m[B^-]^n$$
This definition accounts for the concentrations of each ion in solution. For example, each mole of the generic compound $A_mB_n$ breaks into $m$ moles of $A$ and $n$ moles of $B$.
Quantitative
Relating to the quantity or some measure of something rather than a quality
In science we try to speak of things more quantitatively. Instead of saying that something is "heavy," ask "how heavy is it?" Is it twice as heavy as something familiar, three times? If something gets larger, does it get 10% larger? How much larger. If a sample gets hot, how hot? &c.
Calculating the solubility product
The solubility product constant, $K_{sp}$ for a dissociation reaction of the form
$$A_mB_n \rightleftharpoons mA^+ + nB^-$$
is defined as
$$K_{sp} = [A^+]^m[B^-]^n$$
Example 1
Find the $K_{sp}$ expression for the dissociation of NaNO3.
$$NaNO_3 \rightleftharpoons Na^+ + NO_3^-$$
So the $K_{sp}$ expression is:
$$K_{sp} = [Na^+][NO_3^-]$$
As in algebra, we omit exponents of 1; they are assumed to be there.
Example 2
Find the $K_{sp}$ expression for the dissociation of PbF2
$$PbF_2 \rightleftharpoons Pb^{2+} + 2 F^-$$
So the $K_{sp} expression is:
$$K_{sp} = [Pb^{2+}][F^-]^2$$
Notice the difference between these two examples: Because there are two moles of F- for every mole of PbF2, the concentration of F- is squared in the $K_{sp}$ expression.
Solubility products have been measured and tabulated — you can look them up.
The solubility product constants $(K_{sp})$ of many ionic compounds have been measured and collected in tables. You can download one here, and there are many other on-line resources for the properties of compounds, including $K_{sp}$
The great power of the $K_{sp}$ is that we can use it to predict the concentration of ions in a solution that can't hold any more solute (a saturated solution) or to predict how much more of a solute can be added before precipitation occurs.
Work through the examples below to get a handle on $K_{sp}$ calculations. They're not too difficult and they're pretty handy.
Example 3
Which is more soluble in water, Fe(OH)2 or Fe(OH)3 ? In a saturated solution of each, what molar concentration of Fe2+ or Fe3+ is present ?
$$Fe(OH)_2 \rightleftharpoons Fe^{2+} + 2OH^-$$
$$Fe(OH)_3 \rightleftharpoons Fe^{3+} + 3OH^-$$
Now write the $K_{sp}$ expressions for each; this can be done by just looking at the equation, then look up the known $K_{sp}$ value for each compound:
$$ \begin{align} K_{sp} &= [Fe^{2+}][OH^-]^2 \\[5pt] &= 4.87 \times 10^{-17} \end{align}$$
$$ \begin{align} K_{sp} &= [Fe^{3+}][OH^-]^3 \\[5pt] &= 2.79 \times 10^{-39} \end{align}$$
OK, now we use some stoichiometry. Notice from the first dissociation equation for Fe(OH)2 that one mole of Fe(OH)2 dissociates into one mole of Fe2+ and two moles of OH-, so if we call [Fe] "x", then [OH-] will be 2x. In the Fe(OH)3 reaction we let x = [Fe3+], then [OH-] = 3x, just by looking at the coefficients of the balanced dissociation reactions. So we get:
$$ \begin{align} [x][2x]^2 &= 4.87 \times 10^{-17} \\[5pt] 4x^3 &= 4.87 \times 10^{-17} \\[5pt] x &= \left( \frac{4.87 \times 10^{-17}}{4} \right)^{1/3} \\[5pt] [Fe^{2+}] &= 2.3 \times 10^{-6} \; M \end{align}$$
$$ \begin{align} [x][3x]^3 &= 2.79 \times 10^{-39} \\[5pt] 27x^4 &= 2.79 \times 10^{-39} \\[5pt] x &= \left( \frac{2.79 \times 10^{-39}}{27} \right)^{1/4} \\[5pt] [Fe^{3+}] &= 1.0 \times 10^{-10} \; M \end{align}$$
Now we can answer the questions asked. First, Fe(OH)2 is more soluble in water than Fe(OH)3 because its $K_{sp}$ is 22 orders of magnitude (1022 !) larger. Now in a saturated solution of any salt, the dissolved ions are in equilibrium with the solid sitting at the bottom of the beaker, so the product of the concentrations of the ions in a $K_{sp}$ expression will be exactly the measured $K_{sp}$ that we looked up in the table. Just doing the algebra gives the concentrations of the different oxidation states of iron ions.
Example 4
Predict what will happen when 20 g of NaOH and 13 g of Mg(OH)2 are added to 250 ml of water.
$$2 \, NaOH + MgCl_2 \rightleftharpoons NaCl + Mg(OH)_2$$
Note that the equation had to be balanced with the 2's. Now the solubility rules tell us that sodium hydroxide (NaOH), sodium chloride (NaCl) and magnesium chloride (MgCl2) are soluble, but magnesium hydroxide [Mg(OH)2] is much less soluble. It's $K_{sp}$ is 5.61 x 10-12. That means we can refine our equation with some state labels:
$$2 \, NaOH_{(aq)} + MgCl_{2 \; (aq)} \rightleftharpoons 2 \, NaCl_{(aq)} + Mg(OH)_{2 \; (s)},$$
as long as we allow that even with excess NaOH, so that all of the Mg2+ ions are matched by hydroxides, some of the Mg(OH)2 will be in solution.
Now we have to find out how many moles of reactants we begin with:
$$ \require{cancel} \begin{align} 20 \cancel{\text{ g NaOH}} \left( \frac{1 \text{ mol NaOH}}{40.0 \cancel{\text{g NaOH}}} \right) &= 0.50 \text{mol NaOH} \\[5pt] 13 \cancel{\text{ g MgCl}_2} \left( \frac{1 \text{ mol MgCl}_2}{58.45 \cancel{\text{g MgCl}_2}} \right) &= 0.135 \text{ mol MgCl}_2 \end{align}$$
Now we know from the stoichiometry that all of the MgCl2 will be converted to Mg(OH)2 because we have an excess of soluble NaOH. That means that we easily have two OH- ions for every >Mg2+.
But more than that, for every Mg2+ that does happen to ionize in solution, there will be an excess OH- or two to attach to it, so we'll have precious little ionized Mg(OH)2 around. The solution will contain a charge-balanced mixture of Na+, OH- and Cl- ions, and a white precipitate of insoluble Mg(OH)2 in the bottom of the beaker.
Finally, because the Mg(OH)2 is saturated in this solution, we can calculate the number of free Mg2+ ions. The $K_{sp}$ expression is [Mg2+][OH-]2 = 5.61 × 10-12. Following the calculations in example 3 and substituting x for [Mg2+], we get
$$ \begin{align} [x][2x]^2 = 4x^3 &= 5.61 \times 10^{-12},\\[5pt] \text{so } \; \; [Mg^{2+}] &= 1.1 \times 10^{-4} \; \text{M} \end{align}$$
However, we'd have to consider this to be an upper limit on the concentration of magnesium ions because of the presence of excess hydroxide ions which can pull them quickly back into the precipitate.
Example 5
6.08 × 10-10 moles of iron(II) sulfide, FeS, can be dissolved in a liter of pure water at 25˚C. Calculate the $K_{sp}$ of FeS at that temperature ($K_{sp}$s are temperature dependent).
$$FeS_{(aq)} \rightleftharpoons Fe^{2+}_{(aq)} + S^{2-}_{(aq)}$$
For every mole of FeS that dissolves, we get one mole of Fe2+ and one mole of S2-, so the $K_{sp}$ expression is
$$ \begin{align} K_{sp} &= [Fe^{2+}][S^{2-}] \\[5pt] &= [6.08 \times 10^{-10}][6.08 \times 10^{-10}] \\[5pt] &= 3.7 \times 10^{-19} \end{align}$$
Temperature dependence of solubility
The solubility of some ionic compounds is dependent on temperature. The graph below shows some examples of the temperature dependence of solubility.
In general, we might expect that solubility of a given salt would increase with temperature for two reasons: (1) the increased temperature causes bonds to vibrate more rapidly and with greater amplitude (they stretch out more), leading to increased ionization of difficult-to-ionize compounds; and (2) more rapid atomic and molecular movement in the solution is apt to keep things in solution (things just get bumped around more).
In general, these things are true but some solutes have interesting behaviors that have to be explained.
The graph shows that sodium arsenate (Na2HAsO4) and barium nitrate (Ba(NO3)2) have just the behavior we expect: They become more soluble at higher temperatures.
That's only slightly true for sodium chloride (NaCl), probably because NaCl ionizes so easily even in cold water. There's not much to be gained by heating an NaCl solution.
Ce2(SO4)3·9H2O is called cerium sulfate nonahydrate, which means cerium sulfate with nine water molecules attached. Many compounds exist as hydrates unless they're treated very carefully. Even in solid form they are energetically stabilized by the presence of water molecules. For Ce2(SO4)3·9H2O, heating the solution only causes the molecules that would form that protective hydration shell to jiggle loose, and on average, the core Ce2(SO4)3 is less stable than its hydrated solid counterpart. Ce2(SO4)3 shows retrograde solubility as it is heated; its solubility decreases with increasing temperature.
Solid salts for which these waters of hydration have been removed are called anhydrous salts. You have to be careful with them, because just opening the jar will hydrate them with humidity from the air.
Sodium sulfate, Na2SO4, has truly bizarre solubility as a solution is heated. Up to a temperature of about 33˚C, the waters of hydration remain associated with the compound and it becomes more soluble as it is heated. But above 33˚C, those waters "melt" away and the free Na+ and SO42- are released. These then show a slight retrograde solubility as the temperature is increased.

Most salts are more soluble at higher temperatures, but there are exceptions.
Practice problems
Calculate the concentrations of each dissolved ion in a saturated solution of these salts:
-
$Fe(OH)_2 \phantom{000} K_{sp} = 4.87 \times 10^{-17}$
Solution
Fe(OH)2 ⇌ Fe2+ + 2OH-
For each Fe(OH)2 that dissolves (dissociates), we get two OH- ions.
$$ \begin{align} K_{sp} &= [Fe^{2+}][OH^-]^2 = 4.87 \times 10^{-17} \\[5pt] [x][2x]^2 &= 4.87 \times 10^{-17} \\[5pt] 4x^3 &= 4.87 \times 10^{-17} \\[5pt] x &= \left( \frac{4.87 \times 10^{-17}}{4} \right)^{1/3} \\[5pt] x &= 2.3 \times 10^{-5} \; M \end{align}$$
[Fe2+] = 2.3 × 10-5 M
[OH-] = 4.6 × 10-5 M
-
$Li_3PO_4 \phantom{000} K_{sp} = 2.37 \times 10^{-4}$
Solution
Li3PO4 ⇌ 3Li+ + PO43-
For each Li3PO4 that dissolves (dissociates), we get three Li+ ions.
$$ \begin{align} K_{sp} &= [Li^+]^3[PO_4^{3-}] = 2.37 \times 10^{-4} \\[5pt] [3x]^3[x] &= 2.37 \times 10^{-4} \\[5pt] 3x^4 &= 2.37 \times 10^{-4} \\[5pt] x &= \left( \frac{2.37 \times 10^{-4}}{3} \right)^{1/4} \\[5pt] x &= 9.43 \times 10^{-2} \; M \end{align}$$
[Li+] = 2.83 × 10-1 M
[PO4-] = 9.43 × 10-2 M
-
$PbC_2O_4 \text{(lead oxalate)} \phantom{000} K_{sp} = 8.5 \times 10^{-9}$
Solution
PbC2O4 ⇌ Pb2+ + C2O42-
$$ \begin{align} K_{sp} &= [Pb^{2+}][C_2O_4] = 8.5 \times 10^{-9} \\[5pt] [x][x] &= 8.5 \times 10^{-9} \\[5pt] x^2 &= 8.5 \times 10^{-9} \\[5pt] x &= \sqrt{8.5 \times 10^{-9}} \\[5pt] x &= 9.22 \times 10^{-5} \; M \end{align}$$
[Pb2+] = 9.22 × 10-5 M
[C2O42-] = 9.22 × 10-5 M
-
$Ca_3(PO_4)_2 \phantom{000} K_{sp} = 2.07 \times 10^{-33}$
Solution
Ca3(PO4)2 ⇌ 3 Ca2+ + 2 PO43-
$$ \begin{align} K_{sp} &= [Ca^{2+}]^3[PO_4^{3-}]^2 = 2.07 \times 10^{-33} \\[5pt] [3x]^3[2x]^2 &= 2.07 \times 10^{-33} \\[5pt] 108x^5 &= 2.07 \times 10^{-33} \\[5pt] x &= \left( \frac{2.07 \times 10^{-33}}{108} \right)^{1/5} \\[5pt] x &= 1.14 \times 10^{-7} \; M \end{align}$$
[Ca2+] = 3.42 × 10-7 M
[PO4-] = 2.28 × 10-7 M
-
$CaSO_4 \phantom{000} K_{sp} = 4.93 \times 10^{-5}$
Solution
CaSO4 ⇌ Ca2+ + SO42-
$$ \begin{align} K_{sp} &= [Ca^{2+}][SO_4^{2-}] = 4.93 \times 10^{-5} \\[5pt] [x][x] &= 4.93 \times 10^{-5} \\[5pt] x^2 &= 4.93 \times 10^{-5} \\[5pt] x &= \sqrt{4.93 \times 10^{-5}} \\[5pt] x &= 7.02 \times 10^{-3} \; M \end{align}$$
[Ca2+] = 7.02 × 10-3 M
[SO42-] = 7.02 × 10-3 M
-
$KClO_4 \phantom{000} K_{sp} = 1.05 \times 10^{-2}$
Solution
KClO4 ⇌ K+ + ClO4-
$$ \begin{align} K_{sp} &= [K^{+}][ClO_4^{-}] = 1.05 \times 10^{-2} \\[5pt] [x][x] &= 1.05 \times 10^{-2} \\[5pt] x^2 &= 1.05 \times 10^{-2} \\[5pt] x &= \sqrt{1.05 \times 10^{-2}} \\[5pt] x &= 1.02 \times 10^{-1} \; M \end{align}$$
[Ca2+] = 1.02 × 10-1 M
[SO42-] = 1.02 × 10-1 M
-
Silver bromide (AgBr) is used to make film for black and white photography. Write the chemical dissociation for AgBr in water, and calculate the concentration of Ag+ and Br- in a saturated solution of AgBr.
Solution
You can look up the $K_{sp}$ of AgBr; it's 7.7 × 10-13.
AgBr ⇌ Ag+ + Br-
$$ \begin{align} K_{sp} &= [Ag^{+}][Br^{-}] = 7.7 \times 10^{-13} \\[5pt] [x][x] &= 7.7 \times 10^{-13} \\[5pt] x^2 &= 7.7 \times 10^{-13} \\[5pt] x &= \sqrt{7.7 \times 10^{-13}} \\[5pt] x &= 8.77 \times 10^{-7} \; M \end{align}$$
[Ag+] = 8.77 × 10-7 M
[Br-] = 8.77 × 10-7 M
-
1.0 L of a saturated solution of AgCl is evaporated until only crystalline AgCl remains. The solid matter is weighed and found to have a mass of 3.841 mg. Calculate the $K_{sp}$ for AgCl from this experiment.
Solution
AgCl ⇌ Ag+ + Cl-
First calculate the number of moles of AgCl (FW = 143.32 g/mol).
$$ \begin{align} 3.841 &\times 10^{-3} \, g \, AgCl \left( \frac{1 \; mol}{143.32 \, g} \right) \\[5pt] &= 2.86 \times 10^{-5} \text{ mol AgCl} \end{align}$$
Calculate the concentration of AgCl by dividing by the volume (trivial in this case):
$$ \begin{align} [AgCl] &= \frac{2.86 \times 10^-5 \, mol}{1 \; L} \\[5pt] &= 2.86 \times 10^{-5} \; M \end{align}$$
Now the equation says these ions are present in a 1:1 ratio, so we can calculate the $K_{sp}$.
$$ \begin{align} K_{sp} &= [Ag^{+}][Cl^{-}] \\[5pt] &= [2.86 \times 10^{-5}]^2 \\[5pt] &= 8.18 \times 10^{-10} \end{align}$$
$K_{sp}$ = 8.18 × 10-10
The common-ion effect
Here's a question. What if we try to dissolve two salts in water, both of which contain one of the same ions, a common ion?
This problem pops up now and then, and solubility product constants ($K_{sp}$) can help you sort out what will happen. Lets look at an example:
Imagine we try to dissolve 10 g of Na2SO4 in one liter of water along with 1 g of BaSO4. What will the final concentrations of each ion (Na+, Ba2+, and the common ion, SO42-) be ?

Well, Na2SO4 is a soluble salt, by which we usually mean completely soluble, so we don't need a $K_{sp}$ calculation for that. If we dissolve 10 g of Na2SO4 in water, all of it will dissolve. (There are limits, of course. In fact, only about 140 g of Na2SO4 will dissolve in 1L of water, but that's a lot.)
Now 10 g of Na2SO4 is 0.07 moles of Na2SO4:
$$ \begin{align} 10 \cancel{\text{ g Na}_2 \text{SO}_4} &\left( \frac{1 \text{ mol Na}_2\text{SO}_4}{142 \cancel{\text{ g Na}_2 \text{SO}_4}} \right) \\[5pt] &= 0.07 \text{ mol Na}_2\text{SO}_4 \end{align}$$
Now the question is, given that the solution already has a concentration of 0.07 moles of Na2SO4 per liter, how much BaSO4 can be dissolved?
The solubility equations, both of which contain the common ion, SO42-, are
$$ \begin{align} Na_2SO_4 &\rightleftharpoons 2 \, Na^+ + \color{magenta}{SO_4^{2-}} \\[5pt] BaSO_4 &\rightleftharpoons Ba^{2+} + \color{magenta}{SO_4^{2-}} \end{align}$$
The $K_{sp}$ expression for BaSO4 is
$$[Ba^{2+}][SO_4^{2-}] = 1.08 \times 10^{-11}$$
Now looking at the chemical equations, we see that there are twice as many moles of Na+ as SO42- in the first, and in the second, [Ba2+] = [SO42-], so the total concentration of SO42- ions is
$$\frac{1}{2}[Na^+] + [Ba^{2+}] = [SO_4^{2-}]$$
Now we already know that the concentration of sodium atoms will be
$$[Na^+] = 0.14 \, M$$
Now we make an important approximation. Because $K_{sp}$ for the BaSO4 dissolution is small, compared to 0.14M, the concentration of Na+, the concentration of Ba2+ should be very small. In other words:
$$[Na^+] \gt\gt [Ba^{2+}]$$
So we can make the very good approximation,
$$\frac{1}{2}[Na^+] \approx [SO_4^{2-}] = 0.14 \, M$$
Now, once again we have
$$[Ba^{2+}][SO_4^{2-}] = 1.08 \times 10^{-11}$$
Substituting the concentration of SO42-, we can calculate the concentration of Ba2+ ions:
$$[Ba^{2+}] = \frac{1.08 \times 10^{-11}}{0.14}$$
It is
$$[Ba^{2+}] = 7.7 \times 10^{-11} \, M$$
That's a significant difference from the concentration of Ba2+ we would expect in a saturated solution of BaSO4 without any Na2SO4.
$$[Ba^{2+}] = 3.3 \times 10^{-6} \, M$$
The presence of the common ion, SO42-, "pushed the solubility equilibrium to the left." In case you've already studied it, this is a good example of L'Chatelier's principle of equilibrium.
Example 6
20 ml of 0.005 M KIO3 is added to 5.0 ml of 0.01 M Pb(NO3)3. Identify and calculate the mass of any precipitate that forms.
$$ \begin{align} KIO_3 &\rightleftharpoons K^+ + IO_3^- \\[5pt] Pb(NO_3)_2 &\rightleftharpoons Pb^{2+} + 2 \, NO_3^- \end{align}$$
and the overall double-displacement reaction is
$$2 \, KIO_3 + Pb(NO_3)_2 \rightleftharpoons Pb(IO_3)_2 + 2 \, NO_3$$
Now KIO3 and Pb(NO3)2 are soluble salts, and so is the product KNO3, so we'll assume that they dissociate completely. Lead iodate, however, Pb(IO3)2 is much less soluble. It's dissociation reaction is
$$Pb(IO_3)_2 \rightleftharpoons Pb^{2+} + 2 \, IO_3$$
and the solubility product is
$$K_{sp} = [Pb^{2+}][IO_3^-]^2 = 2.8 \times 10^{13}$$
There's a common ion here: the IO3-. We'll have to keep that in mind when finding the final concentration of Pb2+ in this solution. First, let's calculate the molar concentrations of the reactants. The total solution volume is 0.025 L
The concentration of KIO3 is
$$ \begin{align} 0.02 \cancel{\text{ L sol'n}} &\left( \frac{0.005 \text{ mol KIO}_3}{1 \cancel{\text{ L sol'n}}} \right)\left( \frac{1}{0.025 \text{ L}} \right) \\[5pt] &= 0.004 \, M \end{align}$$
and the concentration of Pb(NO3)2 is
$$ \begin{align} 0.005 \cancel{\text{ L sol'n}} &\left( \frac{0.01 \text{ mol Pb(NO}_3\text{)}_2}{1 \cancel{\text{ L sol'n}}} \right)\left( \frac{1}{0.025 \text{ L}} \right) \\[5pt] &= 0.002 \, M \end{align}$$
The concentration of IO3- ions that would be present due only to the dissociation of Pb(IO3)2 is very small. If we let x = [Pb2+] and 2x = [IO3-],
then:
$$ \begin{align} 4x^3 &= 2.8 \times 10^{-13} \\[5pt] x &= 4.12 \times 10^{-5} \end{align}$$
So [IO3-] = 8.24 x 10-5 M, which is considerably less than the concentration of IO3- from dissolving KIO3, [IO3-] = 4 x 10-3 M (a factor of 100 greater). Because of this, we can make a reasonable approximation that the concentration of IO3- in solution is just 4 x 10-3 M, and it makes the rest of the calculation much easier. We make a similar approximation for [Pb2+]. Some of it comes from soluble Pb(NO3)2 and a much smaller amount from far less soluble Pb(IO3)2 .
Will the Pb(IO3)2 precipitate?
We can predict this by calculating a number usually called $Q$, which is just the $K_{sp}$ expression, $Q = [Pb^{2+][IO_3^-]$, and compare it to $K_{sp}$. If $Q \gt K_{sp}$, the salt will precipitate, and if $Q \lt K_{sp}$, it will not.
In this example,
$$ \begin{align} Q &= [0.002][0.004]^2 \\[5pt] &= 3.2 \times 10^{-8} \end{align}$$
Now Q >> $K_{sp}$ = 2.8 x 10-13, so we predict that a precipitate of Pb(IO3)2 will form. Now to calculate the concentration of Pb2+ that will actually be present in such a solution. Recall that
$$K_{sp} = [Pb^{2+}][IO_3^-]^2 = 2.8 \times 10^{-13}$$
we can rearrange to calculate [Pb2+] using [IO3-] from soluble KIO3:
$$[Pb^{2+}] = \frac{2.8 \times 10^{-13}}{[IO_3^-]^2} = \frac{2.8 \times 10^{-13}}{[0.004]^2}$$
The concentration of lead ions will be
$$[Pb^{2+}]=1.75 \times 10^{-8} \, M$$
Video examples
1. Calculating the solubility of a salt from its $K_{sp}$
Minutes of your life: 3:12
2. Calculating the solubility of a salt from its $K_{sp}$
Minutes of your life: 2:58
3. Using $K_{sp}$ to predict whether a solute will precipitate
Minutes of your life: 3:42
aqueous
An aqueous solution is one in which the solvent is water (root = "aqua"). Typically, but not always, aqueous solutions are ionic salts dissolved in water.

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.