A handy way to model covalent bonding
Lewis dot structures (or just Lewis structures) were developed around 1920 by pioneering chemist Gilbert Lewis, as a way of picturing chemical bonding in molecules.
We draw Lewis structures to
- Discover the bonding arrangement of atoms,
- Discover whether there is any degeneracy of bonding (more on that later),
- Figure out whether a given group of atoms might even bond together to form a molecule at all, and
- Discover clues about the three-dimensional structure of molecules.
In a Lewis structure, every atom is surrounded by dots that represent its valence-shell electrons. So nitrogen (N) would look like this:
![]()
Sometimes we draw the electrons of different atoms with different colors or symbols so we can keep track of them, like this:

OK, let's learn how to use Lewis structures.
Example 1: Methane, CH4
You might have seen the bonding of methane already in the section on covalent bonding. With Lewis structures, we take a trial-and-error approach to figuring out bonding patterns. The goal is to make sure that each atom is surrounded by eight dots (two for hydrogen), representing eight valence electrons, some shared in bonds.
In this structure, the carbon atoms shares one of its valence electrons with each hydrogen,
and each hydrogen shares its single electron with carbon to make a compound with a complete valence shell.
In the figure below, the central carbon is surrounded by a complete octet of eight electrons, and each hydrogen has its capacity, two electrons, by sharing electrons.
Two electrons between atoms indicates a single bond, which can be rewritten, as on the right, by a single bar.
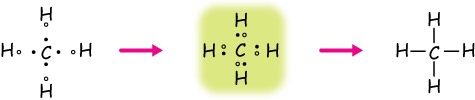
Example 2: Ammonia, NH3
Ammonia is NH3. Nitrogen has five valence electrons and each hydrogen brings one to the molecule. It's easy to see that those three electrons from the hydrogens could complete an octet on the nitrogen: 5 + 3 = 8.
There's a twist in this molecule—a small one—and it gives ammonia some startling properties, some of which are beyond the scope of these notes, but trust me, they're cool.
The figure below shows how to construct the Lewis structure. Start with each atom surrounded by its valence electrons, 5 for N, 1 for H. I like to color each atom's electrons differently so I can keep track of them, but it's not absolutely necessary.
Arrange the atoms by trial and error (and intuition that you'll develop with enough practice) to get a structure in which the sharing of electrons completes the valence shells of all atoms. That's the most likely way the molecule will bond.
One of the pairs of electrons on the nitrogen of ammonia is not bonded. We call that a lone pair. It occupies its own stable orbital, as shown in the stick diagram on the right.

Lewis structures
Lewis structures tell us about the most-likely bonding arrangement and bond types of molecules, but they tell us little about the structure - the 3D shape of the molecule. For that we'll need other skills.
Example 3: Water, H2O
Water is a very important molecule, so it's important to understand its bonding, which will in turn create all of its other properties.
The Lewis structure of water shows that the oxygen atom has two lone pairs.
Those lone pairs, together with the large difference in electronegativity between oxygen and hydrogen, give water one of its most important properties, its strong polarity.
In the rendering on the right below you can see that water, which actually is a bent planar molecule (again, you wouldn't necessarily know this just from the Lewis structure), has a negative end and a positive end.
More correctly, it has one end that is more negative than the other (called δ-) and one more positive (called δ+). The unbonded electron pairs create a region of dense negative charge. And because oxygen holds the bonding electrons of the H-atoms tightly to itself, the H-atoms are essentially bare protons hanging off the oxygen.
The polarity of water and its ability to hydrogen bond gives water some of the properties that are deeply intertwined with the chemistry of living things on Earth. It's for that reason that it's difficult for us to conceive of life on another planet without water – but you never know ...

Example 4: Double & triple bonds
Lewis structures can show us when double and triple bonds are most likely, or perhaps the only kind of bonding that make a molecule possible. Here are some Lewis structures that contain double and triple bonds (and indeed the real molecules do, too).
The double bonds in carbon dioxide, CO2, are what make it a linear, non-polar molecule, and that structure, in turn, gives it most of its properties.
CO2 solidifies, for example, at about -57˚C, and the liquid only exists if the gas is placed under about 5 atmospheres of pressure. The triple bond of nitrogen gas, N2, is very strong. Although our atmosphere is mostly nitrogen in the form of N2, most organisms on Earth can't use it in that form because they can't break that bond. They require other sources of the crucial element.

Example 5: Degeneracy
Some Lewis structures will lead to bonding that is ambiguous. A double bond might be present between an atom and one or more other equivalent partners. Which one to choose? This is called a degeneracy, and it turns out that nature tends to pick both, neither, and a combination of the two bonds.
As an example, let's look at the Lewis structure of nitric acid, HNO3. First the atoms with their valence electrons:

Now we can arrange the bonds in two ways. In both, all atoms have a full valence shell. Here they are:

Take a minute and convince yourself that every atom (except the hydrogen) has an octet of electrons in its valence.
Here are the two structures in stick form:

So which one does nature pick? Well, it turns out that whenever we have two equivalent structures like this (we have two degenerate structures or a degeneracy), nature picks a combination of both, and we're better off writing the two bonds more like 1-1/2 bonds, like this:

Notice that the red oxygen is different than the other two. It's bound to a hydrogen and the electronegativity difference makes this bond more ionic than covalent. The result is that the hydrogen can detach as a bare proton quite readily, leaving a NO3- ion behind. That's why HNO3 is an acid.
Example 6: Carbonate ion, CO32-
Now let's see how a molecular ion, the carbonate ion, CO32-, can bond stably. When we work with ions, we begin with the usual number of valence electrons of a neutral molecule, in this case four for carbon and six for each of the three oxygens. But this is a 2- ion, so we'll add two electrons to the neutral mix to give it that -2 net charge.
Those last two electrons can be filled in anywhere they're needed to form a full valence. We start with the raw materials:

Now put them together and use the two extra (red) electrons to fill in any gaps in order to form full valence shells for every atom.
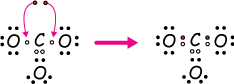
Finally, notice that this is another degenerate structure. There are two other equivalent places to put our double bond, like this:
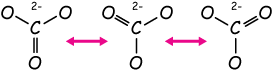
In this case, the reality is that each C-O bond is equal, stronger than a single bond, but weaker than a double bond. We might write the structure of CO32- like this:

Here I've left off the lone pairs of the oxygen atoms. There might be a context in which we'd want to show those, but most of the time it's quicker to omit them.
Molecular ions
Many molecules for which stable valence shells cannot be built from the number of electrons present on the neutral atoms that compose them can be made stable if electrons are lost are gained. These are molecular ions.
Some important molecular ions are OH-, NH4+, SO42-, PO43-, CO32-, COO- & CN-
Example 7: Sulfate ion, SO42-
The sulfate ion, SO42-, is a very interesting exception to many of our assumptions about bonding. In fact, it caused a lot of argument in the chemistry community early on. At first glance, with sulfur and oxygen both holding six valence electrons, we might draw a Lewis structure like this:

The problem is that the two extra charges needed to stabilize this molecule are localized on the sulfur atom. Nature tends to spread that extra charge out, and sometimes at a cost that can contradict what we've already learned. In this case, it's the octet rule. In fact, SO42- tends to bond more like this:

Now the extra charges are a little more spread out, and we can see that there's nothing special about our double bond locations, which means resonance structures and even more spreading of charge. But the twelve electrons around the sulfur are troubling. Remember that sulfur has d-electrons, and some of those are used as valence electrons.
Overall this structure is more stable than the all singly bonded one.
Here are all of the resonance forms of SO42-, and they lead to an ion with four identical bonds that are somewhere between double and single in strength.

It was a measure of the S-O bond length in SO42- that led to a deeper investigation of the bonding.
You shouldn't feel like you ought to have recognized this case. It took some Nobel-prize winning chemists, including Linus Pauling, some time and significant argument to uncover the truth. It says a lot about the nature of the electron, if you think about it.
Some other exceptions
The sulfate ion (above) is one case of an exception to the octet rules. There are others. I'm not sure there's any point in memorizing such exceptions; better to know that they exist and be wary of them. Know some of the signs that an exception might exist. Here are a few examples.
Phosphorus pentachloride, PCl5
Phosphorus pentachloride (PCl5) is an exception to the octet rule. You can see the Lewis structure of PCl3 in the practice problems below. Because a chlorine atom only needs one electron to complete its valence shell, it shares one and only one electron with phosphorus, so in PCl5, phosphorus is surrounded by a total of ten electrons. It does this by using its d-shell electrons.

A more 3-dimensional structure is shown on the right. Three chlorines are in a plane (blue triangle) and the line containing the other two cuts through the center of that triangle and is perpendicular to it. The arrangement is called a trigonal bipyramid.
Sulfur hexafluoride, SF6
For similar reasons, sulfur can bind to six fluorine atoms with single bonds in a square bipyramid arrangement.

Xenon hexafluoride, XeF6
Finally, we don't normally think of noble gases interacting with anything, let alone forming bonds. But it turns out that if the noble gas is big enough, like xenon (Xe), the d-orbitals can allow bonds to form. Xenon forms a couple of covalent compounds, one of which is XF6.
XF6 looks just like SF6, but the central Xe is now surrounded by 14 electrons.

Practice problems
Roll over the boxes to see the answers - but not before you've tried it yourself!












When it's too close to call: Formal charge
Sometimes it's difficult to tell which of two possible Lewis structures of a compound represents the actual bonding of the molecule. In those cases we resort to calculating what's called the formal charge of each atom. Formal charge is just a way of bookkeeping that helps us to decide which of multiple Lewis structures is the likely true bonding arrangement of a covalent molecule. The sum of the formal charges, with a couple of extra rules, will help us to decide which of multiple-possible valid Lewis structures is likely to be the correct one. Here's how it's done.
Calculating formal charge
For each atom
- Count the number of valence electrons of the neutral atom.
- Subtract the number of non-bonding electrons (usually in lone pairs).
- Subtract the number of bonds shared by the atom.
Example: CH4 (methane)

The carbon in CH4 has four electrons as a neutral atom. It has no lone pairs, and it shares four bonds, so the formal charge is zero. Each hydrogen atom has one electron as a neutral atom, no lone pairs and shares one bond, for a formal charge of zero. All atoms in the molecule have zero formal charge, the "happiest" situation for any molecule.
Example: H3C(CO)CH3, (acetone)

The central carbon has a formal charge of 4 (valence electrons) - 0 (lone pairs) - 4 (bonds) = 0. The oxygen has a formal charge of 6 - 4 - 2 = 0 (same ordering of terms). Each of the methyl (CH3) carbons has a formal charge of 4 - 0 - 4 = 0
Distinguishing between two valid Lewis structures
Here is an example of a case where we can find two valid Lewis structures for a compound, fulminic acid (HCNO). We can use formal charges to decide which is most likely to be the actual arrangement of atoms. Here are the structures:

Now let's calculate the formal charges of the lower structure, using double-bonds:

Note that the carbon has a formal charge of -1 and the nitrogen a charge of -1. The formal charges of the structure with the triple bond look like this:

Here, the oxygen — the most electronegative element in the molecule — has the negative charge, and the nitrogen retains its +1 charge. This structure is more likely to be the correct one, because the negative charge is on the most electronegative element of C, N and O.
The Lewis structure most likely to represent the actual bonding arrangement is the one in which all formal charges are the closest to zero.
If two structures have similar formal charges, the one in which a negative charge lies on the most electronegative atom wins.

Example: SO3
Given that sulfur is in the third row of the periodic table, and can thus accommodate more than an octet of electrons when bonded in a molecule, there are a number of possible Lewis structures for SO3 that might work. Fortunately, experimental evidence confirms that one of them is the actual structure. Formal charge results are consistent with that structure, too. Here are the Lewis structure possibilities:

Here are the formal charges on each atom for each bonding arrangement. The fully double-bonded structure (right) has the lowest formal charges on each atom. Even though sulfur has a bonded valence of 12 electrons, this is still the most stable structure. A few elements in the third row of the periodic table, plus a great many elements with d-electrons, are capable of this.

This bonding arrangement of SO3 is confirmed by experiment, which shows that its structure is trigonal-planar (a flat molecule with oxygen atoms at the vertices of an equilateral triangle. VSEPR theory predicts that the 1- and 2-double bonded structures would be a little different (but distinguishable).
While formal charge assignment isn't too useful in other areas of chemistry, it can be really enlightening when questions about bonding like this need to be resolved.

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.