A brief overview of "thermo"
By now you may have spent some time thinking about chemistry in a microscopic way: one atom or molecule at a time, or thinking about how individual atoms/molecules move and interact. Now we turn to the properties that matter has because we put moles of it together.
It turns out that the random nature of atomic and molecular movement leads to some very predictable properties of bulk matter. That's what we call matter when we move beyond just a few molecules to a great many, like a mole or more.
The word thermodynamics refers to the movement (dynamics) of heat (thermo). There is much to thermodynamics, but in a broad sense, it's about the movement of heat (which is atomic and molecular kinetic energy), storage of energy as chemical potential energy, and the doing of work.
This section is intended to teach you some of the language of thermodynamics and to introduce some of the key concepts – but it's just an introduction.
Thermodynamics can be tricky to learn, as Physicist Arnold Sommerfeld suggested ...
Thermodynamics subjects
Here is a basic breakdown of the most important subjects in thermodynamics. They each have (or will have!) their own page on this site. Briefly, your path through "thermo" will be to fully understand the nature of heat and work, and how they change, including the sign conventions (see below). Then you'll learn about entropy (a measure of disorder) and the laws of thermodynamics. Finally, you'll put heat and work together in different situations to form important state functions like the Gibbs energy function, which can predict whether a reaction or process will be spontaneous in given situations.
| Laws of thermodynamics |
State functions |
||
| Temperature | Vapor pressure | 1. Conservation of energy. | Enthalpy (H) |
| Heat capacity | Thermo in equilibrium |
2. Entropy increases | Entropy |
| Phase transitions | Thermo in kinetics |
2. Absolute entropy | Gibbs Energy (G) |
| Heat and Work | Helmoltz Energy (G) | ||
| Heat transfer |
A system – definition
For our studies of thermodynamics we will call a system the part of the universe that is being studied. For example, a solution in a beaker or a gas in a closed container can be a system.
The surroundings are everything else around the system that can interact with it. For example, a system consisting of a solution in a beaker can interact with its surroundings, the table top and the atmosphere, by exchanging heat (see heat transfer). In chemistry, the system is usually the reactants and products of the reaction under study, and the surroundings are the container(s) and everything else outside.
A system can be the contents of a beaker, a room or the whole universe. What's important is to clearly define what you mean by system in each circumstance or experiment. Some examples are:
- A person, her clothing (insulation), anything she's touching (heat can transfer through contact), and the air near her body. Air far away has little effect on the person, so we could probably put a limit on how far our system extends.
- A turkey in the oven, the pan and the air around it. We could probably neglect anything outside the oven because the oven is well-insulated and there isn't much transfer of heat to or from the outside.
- A gasoline engine, the gas in the tank, the air surrounding the engine and flowing past it (flowing air transfers heat faster than stagnant air), the cooling fluid circulating through it and the exhaust system, which removes a lot of the heat of combustion of gasoline.

Properties of a system
Intrinsic and extrinsic properties
Some properties of matter in a system depend on how much matter is there, and others are simply properties of the kind of matter. We call these extensive and intensive properties, respectively.
Extensive properties depend on the amount of matter present. For example, if we double the number of atoms of a certain substance, we double its mass, an extensive property. On the other hand, if a substance has a certain density (mass per unit volume, an intensive property), and we double the amount of it, the density is the same.
Here are some examples of extensive properties. You might not know what some of these are right now, but hopefully, you will some day soon.
- energy
- momentum
- number of moles
- length
- charge
- entropy
- mass
- weight
- Gibbs energy
Intensive properties do not depend on the quantity of a substance present, but are physical properties of the substance. When the amount of a substance doubles, its extensive properties double, but its intensive properties do not.
Here are some examples of Intensive properties. Ductility and malleability are the ability of a material to be stretched into a wire and flattened into a sheet, respectively.
- concentration
- hardness
- melting point
- temperature
- ductility
- malleability
- resistivity
- boiling point
- viscosity
- elasticity
- vapor pressure
- specific heat
If two identical systems are brought together, the extensive properties double, while the intensive properties remain the same.
Intensive properties depend on the type or identity of the matter that composes a system.
Extensive properties depend on the amount of substance present.
Isothermal systems & processes
Isothermal literally means "same temperature." Mathematically, we say that an isothermal process is one in which $\Delta T = 0$, where $T$ stands for temperature.
We're really only interested in "processes" here. A process indicates that some thing happens to something, like a chemical reaction or the functioning of an engine or generator. We usually aren't too concerned with things that just sit there and do nothing, unless it's a teenager.
As you will learn, some chemical reactions are exothermic, meaning that they give off heat as they proceed. Some are endothermic, meaning that as they progress, they remove heat from the surroundings. If you touched a beaker in which an endothermic reaction was going on, it would be cold, while an exothermic reaction in liquids would produce a hot beaker.
Sometimes it is advantageous to keep the temperature constant. You might imagine keeping our liquid reaction beaker in a large water bath held at a fixed temperature by a thermostat.
If the water bath is large compared to the reaction mixture, it will soak up extra heat from an exothermic reaction or give heat to an endothermic reaction
without really reflecting it in a large temperature change – because the bath is just so big compared to the beaker.

A temperature-controlled water bath like this is often used to maintain isothermal ($\Delta T = 0$) conditions.
Image: Whipmix.com
The system in the water bath would be the beaker and its contents, the air (if there was no lid) and the water bath. To whatever fraction of a degree we could set the water-bath temperature, the reaction process would be isothermal.
Exothermic processes give off or emit heat to the surroundings.
Endothermic processes require heat from the surroundings in order to occur.
Adiabatic systems & processes
An adiabatic process (a'·dee·uh·bat'·ck) is one in which there is no transfer of heat between the system and its surroundings at all. While this is never entirely possible, it's a very important theoretical book-end for many of the things that we'll learn in thermodynamics, so it's worth thinking about.
The word adiabatic comes from a sandwich of three Greek words, and roughly means "not to be passed through," as in a wall.
In practice, we encounter adiabatic systems and processes in two ways. First, we can make an adiabatic system by surrounding it with enough insulation that there is no measurable heat transfer. What happens inside the insulating box stays in the insulating box. An example of this is just a Styrofoam cooler.
Although there is ice inside, the box doesn't feel cold because it isn't drawing in a lot of heat from the surroundings. It does, but the rate is very slow, and the thicker the box, the slower the heat transfer.
More importantly, processes are approximately adiabatic if they occur so rapidly that there is little time for heat to be transferred. One example might be an explosion of ignited gasoline inside of an engine cylinder. Most (but never all) of the energy goes into the work of moving the piston, but processes that happen right after the explosion are non-adiabatic (combustion engines are less than 50% efficient at converting the energy contained in the bonds of gasoline into movement of the engine parts; the rest is radiated away as heat).
We usually use the letter $q$ for heat, so in an adiabatic process, $\Delta q$.
In an isothermal process, $\Delta T = 0$. In such a process we need a large "bath" of matter that lends or absorbs so little heat compared to its large mass that its temperature doesn't change.
In an adiabatic process, $\Delta q = 0$. In such a process we need to insulate the system from the surroundings so that no heat can flow between them.
Thermodynamics is about the movement of heat (to which we've alluded above) and the concept of work (below).
Heat is covered in detail in another section, so I'll just provide a brief definition here.
Heat is the random movement of atoms and molecules, the movement of atoms within molecules (the vibration of bonds), and the rotation of molecules about their centers of mass.
Atoms
For example, in a container of gaseous argon (Ar), heat is the translational movement of argon atoms in three dimensions in the container.
The more heat we add, the faster, on average, the atoms move, and the higher the temperature we can therefore measure.
Molecules
Now consider a gas of molecules like CO2 (O=C=O). Heat in this case means not just the translational motion (up-down, in-out, and side-to-side) of the whole CO2 molecule, but also the vibration of its two double bonds and the rotation of the molecule about its three-dimensional axes.
To some extent, which will be explained more in the section on heat, rotational motion of molecules also contributes to our measure of heat.
In thermodynamics, when a system absorbs heat, the heat is positive (+q) and we call the process endothermic; and when the system emits or gives off heat, that heat is negative, and we call such a process exothermic.
Translation
Translation is motion in one or more of the three Cartesian (x, y, z) directions, or a combination of them, without any rotation.
The concept of work completes the picture of how energy flows in all systems. In physics, we know that we can do work to lift an object to a height, at which it has a potential energy (due to gravity) exactly equal to the work we did. And in turn that potential energy can be converted to kinetic energy – the energy of motion.
In chemical systems, work is generally done when a system, such as a gas, expands against a force or is compressed under one. For example, when we heat a container of gas with a movable piston that keeps it at a constant pressure (diagram), its temperature rises, and its volume must also rise:
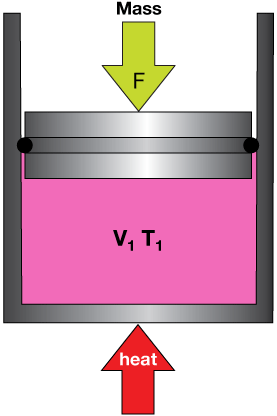
The pressure is kept constant because pressure is force divided by area. The area of the piston and the downward force due to the fixed mass on top of it are both constant in this experiment.
The final state of our system is shown here. The gas has expanded because at a higher temperature, faster speeds, more energetic collisions will push against the piston until the volume increases so much that the number of gas-particle collisions with the piston is smaller than the starting state, but the overall force per area is the same.

In this case, we say that the gas did work on the surroundings by expanding. If we were to have done the experiment in reverse, the surroundings would have done work on the system.
by convention, work done on the system is negative (-w) and work done by the system is positive (+w).
In thermodynamics, we call such expansion and contraction of gases "pressure-volume" work or "PV work."
Heat & work: Sign convention
You can think of a system as being "self-centered." It's all about the world from the point of view of the system
If $q \gt 0$, ($+q$) heat was added to the system in a process (endothermic)
If $q \lt 0$, ($-q$) heat was removed from the system and added to the surroundings by the system (endothermic)
If $w \gt 0$ ($+w$), the system did work on the surroundings, and
if $w \lt 0$ ($-w$), the surroundings did work on the system.
Equivalence of heat and work
Doing work on a system or adding heat to it are completely equivalent. The total energy input into a system is the heat it receives plus the work done on it; it's part of the principle of conservation of energy.
Prescott Joule, one of the early developers of thermodynamics, performed an experiment that showed that mechanical work done on a container of water produces a temperature rise in the water equivalent to the amount of heat needed to cause the temperature change.
In Joule's experiment, weights of known mass were dropped a known distance, thus producing a known amount of kinetic energy that produced mechanical stirring of a quantity of water. A temperature rise roughly equivalent to that amount of energy as heat was then observed in the water.
It's important to remember as you move through thermodynamics that heat and mechanical work are equivalent, just as work, potential energy and kinetic energy can be interchanged in mechanical systems.

As you begin to study state functions, you'll begin with the internal energy change of a system, which is the sum of the changes in heat and work.
State functions
In later sections we'll learn in detail about state functions. Although you've probably got a lot of work to do before you tackle state functions, they are extremely important in chemistry and physics, and the epitome of thermodynamics.
A state function is a mathematical expression (a function) that depends on the flow of heat and work in a system, while holding some variables (like pressure, temperature or the number of moles of particles) constant.
State functions are called state functions because their values depend only on the current state of the system, a state that can be specified by knowing the pressure, temperature, volume an number of moles of substance.
They don't depend at all on how the system got from one state to another, or how it got to its current state, or the path the system took or was put through to go from one state to another.
Some of the most common state functions are
- Internal energy (U)
- Enthalpy (H)
- Entropy (S)
- Gibbs energy (G)
- Helmholtz energy (A)
We will most commonly be calculating differences in state functions, denoted by the Greek letter delta, Δ, which means "change in." So ΔH is the change in the enthalpy state function, and so on.
Right now you probably don't know what these things are, but not to worry, they will become clear with time and in the next few sections on thermodynamics.
The Greek alphabet
| alpha | Α | α |
| beta | Β | β |
| gamma | Γ | γ |
| delta | Δ | δ |
| epsilon | Ε | ε |
| zeta | Ζ | ζ |
| eta | Η | η |
| theta | Θ | θ |
| iota | Ι | ι |
| kappa | Κ | κ |
| lambda | Λ | λ |
| mu | Μ | μ |
| nu | Ν | ν |
| xi | Ξ | ξ |
| omicron | Ο | ο |
| pi | Π | π |
| rho | Ρ | ρ |
| sigma | Σ | σ |
| tau | Τ | τ |
| upsilon | Υ | υ |
| phi | Φ | φ |
| chi | Χ | χ |
| psi | Ψ | ψ |
| omega | Ω | ω |
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.


