Life on Earth is carbon-based
All life we know of on Earth is mostly based on molecules containing the element carbon. Molecules containing carbon are usually referred to as organic compounds; their chemistry is organic chemistry.

Carbon has a total of six electrons. Two are buried in its inner electron shell, leaving four that can bind to other atoms. Except in very special circumstances, carbon always forms four bonds. Because the electrons in bonds repel each other as much as possible, the bonds of carbon tend to arrange themselves in a tetrahedron, like in methane (CH4), shown here:

The central carbon, in this case, is surrounded by four hydrogen (H) atoms. Except when it binds to another atom with a double or triple bond, carbon "centers" in molecules always have this tetrahedral shape.
In this section, we'll just set up the various types of molecules that are involved in living things, put them in some categories, and set up the basic language of biochemistry. Follow the numerous links below to specific molecules and classes of molecules.
Representations of organic molecules
Biological molecules are typically (though not always) very large and complicated, so we need to have some shorthand methods of drawing their structures. You will encounter many different kinds of representations of biological molecules along your way. Each has its strengths in illustrating a particular important feature of a molecule. On the left below is a nice hand sketch (a ribbon diagram) of the enzyme triose phosphate isomerase. It very nicely shows the underlying carbon-backbone
structure of many proteins, with alpha helices (the spring-looking things) and beta sheets (green). In the center are two images of short lengths of DNA molecules. The black one shows the major bonds as "sticks." While the other DNA image, a space-filling model, is more realistic, the stick model can sometimes be clearer to interpret. The image on the right is a stick diagram of sucrose (table sugar). Most small molecules are drawn in a similar fashion.

Common abbreviations
Biology and biochemistry is repleat with abbreviations, owing to the complexity of many of the names of complex molecules. You'll need to learn them and become fluent with them. This table might help you become familiar with them, and to refresh your memory when you need it. Follow the links to more detailed information.
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| ATP, GTP | adenosine triphosphate, guanosine triphosphate |
| ADP | adenosine diphosphate |
| NADH | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| FADH | flavin adenine dinucleotide |
| A, T, G, C, & U | adenine, thymine, guanine, cytosine, & uracil, the DNA bases |
Categories of biological molecules
| Proteins | Nucleic acids | Lipids | Carbohydrates | Small molecules |
| Long polymer chains of 20 types of amino acids | Long & short chains of nucleic-acid bases form DNA, RNA; also ATP, GTP, ... | Phosphate or other charged "head" group with long hydrophobic tail(s) | Long & short chains of sugar molecules like glucose, fructose; starch | Various molecules such as hormones, vitamins, neurotransmitters, porphyrins |
| Constitutes most of the struction & function of living things; enzymes | Information storage (RNA, DNA), structure, enzymes | Energy storage, insulation (thermal & electrical), cushioning, membranes | Energy source, energy storage, structure | Signals & cofactors |
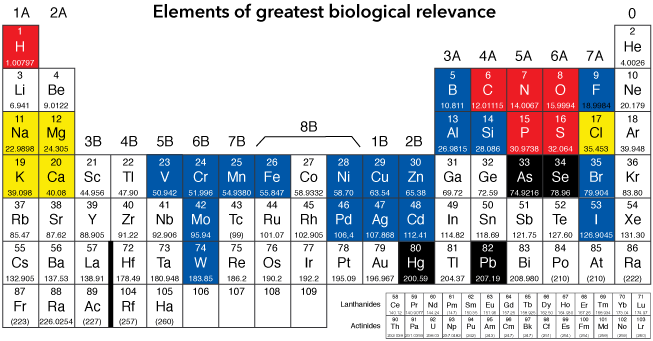
Periodic table of biologically-relevant elements
This periodic table shows the elements of greatest biological significance. The elements in red are the major building blocks of all biomolecules: proteins, nucleotides, carbohydrates and lipids, and most smaller molecules of biological relevance. The blue elements are metals and other elements found in biomolecules and necessary for many functions.
Yellow elements are important ions, for example, in the transmission of nervous signals. The black elements are significant bio-toxins, but many elements, with enough exposure, can harm living organisms.
![]()
The elements carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur are by far the most numerous constituents of biological molecules. Carbon is the atom that determines whether we call a molecule "organic." DNA and RNA comprise nitrogen and phosphorus atoms in every base; nitrogen is a key atom of the amino group of amino acids.
These are truly the atoms of life, and indeed we look for them to be present as a precursor for life (as we understand it to be possible) other places in the universe.
![]()
These important ions are crucial to all kinds of life on Earth. The are important in maintaining charge balance (or sometimes designed imbalance) of charge on two sides of a membrane, and they are the carriers of electric charge in nervous impulses in organisms with nervous systems.
![]()
The blue-hilighted elements are the second-tier atoms of life. They are involved in many ways in the biochemistry of life. They are ions and charge carriers and metallic cofactors in enzymes, among other roles. Most, particularly the metals, are toxic at very high concentrations, but necessary at lower concentrations.
![]()
While many elements are toxic to life on Earth, particularly at high concentrations, these four, arsenic, selenium, mercury and lead, are particularly dangerous, even at relatively low concentrations.
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2016-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.
