This section follows from the electrochemistry section. You should know something about oxidation, reduction, oxidation numbers and redox equation balancing before learning the material in this section. It's all key information that you'll need before moving on.
In this section we'll extend what we learned about conceptual electrochemistry to doing stoichiometry calculations with reactions that involve transfer of electrons between reactants.
We can tie stoichiometry to electric current through electrochemical cells.
What we develop in this section will help us to combine the measurable electric current flowing through batteries, electrolytic cells and fuel cells, with the stoichiometry of redox reactions. In a balanced redox reaction, we understand the number of electrons transferred per mole of any given reactant or product, thus current (the number of charges flowing by a point per unit time) can be used to calculate disappearance of reactants and appearance of products.
We'll rely here on one important quantity, called the Faraday constant, F = 96,485 C/mol. It's easy to derive, so we should do so.
The Faraday constant is just the amount of total charge in a mole of electrons, in SI units of Coulombs per mole (C·mol-1). The charge on one electron is -1.602 x 10-19 C, so a mole of electrons has a total charge of
The Faraday constant is often rounded to 96,500 C/mol.
Because one Ampere ("amp", A) of electric current is the equivalent of 1 C of charge flowing by a point in a circuit every second, we can relate the amount of current over time to the number of moles of electrons transferred.
SI stands for Système international (of units). In 1960, the SI system of units was published as a guide to the preferred units to use for a variety of quantities. Here are some common SI units
| length | meter | (m) |
| mass | Kilogram | (Kg) |
| time | second | (s) |
| force | Newton | N |
| energy | Joule | J |
The Faraday constant is F = 96,485 C/mol
It is the amount of charge on one mole of electrons. Because 1 Ampere (A) = 1 Coulomb per second (1 C·s-1), alternative units of Faraday's constant are A·s·mol-1
Form all possible spontaneous electrochemical cells (batteries) with these half reactions. Calculate the cell potential of each and balance the redox reaction.
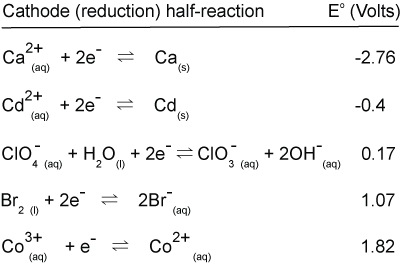
It is possible to run a non-spontaneous electrochemical reaction by applying an external potential (voltage) using a battery or power supply. In this way we can achieve things like decomposing compounds (like splitting water into hydrogen and oxygen, (electrolysis, below), or electroplating, deposition of layers of metal atoms onto various substrate surfaces.
Let's begin with electrolysis. The overall reaction is
A look at the oxidation numbers,
![]()
shows that water is both reduced and oxidized in this reaction:

We can balance the missing atoms in these half reactions by adding H+ to the oxidation reaction and OH- to the reduction. Then we can balance the charges by adding electrons, four to the oxidation and two to the reduction.

These will be the balanced oxidation and reduction half reactions we'll use in the electrolytic cell below.
We can recover our overall equation from the half reactions by multiplying the reduction half-reaction by 2 (to balance the electrons between the two half reactions, and adding (noting that H+ + OH- → H2O).

The half-cell potentials of the half reactions, as written above, are E˚ = -1.23 V and E˚ = -0.83 V, respectively, making this a non-spontaneous cell overall. We can, however, drive the cell in the direction written by applying an external potential using a battery or power supply.
In the apparatus below, both metal electrodes are submersed in water. Because the products, H2 and O2 are gases, two inverted tubes, initially filled with water before inversion, are suspended, one to each, over the electrodes. Any gas evolved at the electrode surface will rise up into the appropriate tube, displacing the water inside. That way, we'll see our product gases forming, and capture them for use later.

When the power supply is turned on, water is reduced at the cathode, the negative terminal and a source of electrons, and it is oxidized at the anode, the positive electrode and a sink for electrons.

Very often it is desireable to affix a thin coating of one metal on another. This can accomplish one or more of several goals, including:
The
The
The

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.