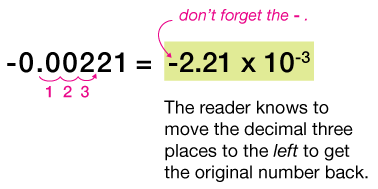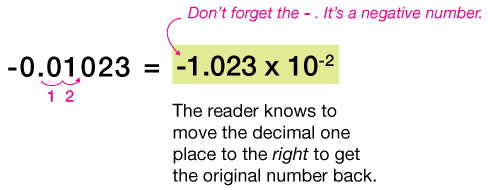Why Scientific Notation?

In the sciences, we often deal with numbers that can differ by many orders of magnitude, that is, by powers of ten. For example, the sun is about $148,986,000,000$ meters away from Earth, but the distance across one of the protons that exist in the plasma of the sun is about $0.000000000000001$ meters. That's a lot of zeros to keep track of and the difference between those numbers is immense. Scientific notation allows us to express those numbers as $1.489 \times 10^{11}$ m and $1 \times 10^{-15}$ m, respectively — less writing and fewer zeros to keep track of, for sure. But you'll also see that scietific notation can make multiplication, division and raising to powers much easier, too.
The convenience of powers of ten
| $10^9$ | $1,000,000,000$ | Giga (G) | |
| $10^8$ | $100,000,000$ | ||
| $10^7$ | $10,000,000$ | ||
| $10^6$ | $1,000,000$ | Mega (M) | |
| $10^5$ | $100,000$ | ||
| $10^4$ | $10,000$ | ||
| $10^3$ | $1,000$ | Kilo (K) | |
| $10^2$ | $100$ | ||
| $10^1$ | $10$ | ||
| $10^0$ | $1$ | ||
| $10^{-1}$ | $0.1$ | deci (d) | |
| $10^{-2}$ | $0.01$ | centi (c) | |
| $10^{-3}$ | $0.001$ | milli (m) | |
| $10^{-4}$ | $0.0001$ | ||
| $10^{-5}$ | $0.00001$ | ||
| $10^{-6}$ | $0.000001$ | micro (μ) | |
| $10^{-7}$ | $0.0000001$ | ||
| $10^{-8}$ | $0.00000001$ | ||
| $10^{-9}$ | $0.000000001$ | nano (n) |
The chart on the left shows several powers of ten, from $10^9$ to $10^{-9}$, and their translations into standard integer or decimal notation.
You can think of the power of ten as an instruction about where to move the decimal. If you begin with the digit $1$, which is the real number $1.0$, then $10^9$ means
$$10^9 = 1,000,000,000$$
Likewise, beginning with $1.0$, if $-9$ is the power of $10$ ($10^{-9}$), that means
$$10^{-9} = 0.000000001$$
The common metric system prefixes are listed in
These powers of ten form the backbone of scientific notation. Now let's look at the anatomy of a number written in scientific notation.
Orders of magnitude
In science and mathematics, a power of ten difference is called an order of magnitude. For example, $100 \; (10^2)$ and $1,000,000$ ($10^6$) differ by four orders of magnitude.
An amazing film: "Powers of Ten"
Take a few minutes to watch this short film, "Powers of Ten", produced in 1977 by extraordinary designers and educators, Charles and Ray Eames. As you watch it, remember that it was filmed (yes FILMed) before we had much in the way of digital graphics capabilities. Seen in that light, it's a truly amazing piece of work, and it will really help illustrate the meaning of orders of magnitude.
Anatomy of a number in scientific notation
Now we can make a very simple extension of the powers of ten above to create what we call scientific notation. On the right are a couple of examples of numbers in scientific notation.
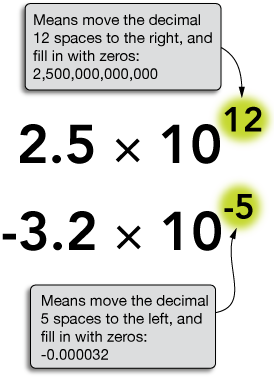
$2.5 \times10^{12}$ means to take $2.5$, move the decimal 12 places to the right and fill in the open gaps with zeros to make 2,500,000,000,000 or 2.5 trillion. In scientific notation, the number on the left is always a single digit followed by a decimal point and the correct (see significant figures) number of digits after the decimal.
$-3.2 \times 10^{-5}$ means to take the negative number $-3.2$, and move the decimal 5 places to the left, filling in the open gaps with zeros to make $-0.000032$. Notice that the minus sign to the left of the 3.2 has nothing to do with the exponent, and simply indicates a negative number.
We would write -2.5 trillion as $-2.5 \times 10^{12}$.
Scientific notation: form
In scientific notation, the number on the left is always a single digit followed by a decimal point and the correct (see significant figures) number of digits after the decimal.
Converting numbers to scientific notation
Here are a few examples of how to convert numbers, big and small, to scientific notation. This is one of the tasks you may have to do most, so it's a good one to learn.
Example 1: 12,000,000
First, there is an implicit (not shown but there) decimal after 12,000,000, and our goal is to put it after the 1 and before the 2:

Now we simply count how many spaces from the right (the original decimal) that is:

Now it's a simple matter to form the number in scientific notation. It's 1.2 with 6 zeros (the 7th place is taken up by the 2):
![]()
Note that the number in scientific notation is really a kind of recipe for recovering our original number: "Take 1.2 and move the decimal 7 places to the right." When we're forming the scientific notation, the move is in the opposite direction, but that's only to form 1.2 x 107 in the first place.
Example 2: 232,124.5
This example is similar to the first, except that there is actually a decimal in the original number.

To move the decimal between the 2 and the 3, we'll need to move it five places to the left:

Now our number in scientific notation is
![]()
In this case we have carried a lot of digits from the original number. Those should be carried as long as they're significant according to the rules of significant digits.
Example 3: 0.000323
Here's our first example of a number less than one. We'll want to move the decimal between the 3 and the 2:

That means moving the decimal four places to the right:

to get our number in scientific notation:
![]()
Remember that this number is a recipe for recovering our original number. It says, "move the decimal four places to the right, filling spaces with zeros, to get the original number 0.000323."
Example 4: -0.0000044
Our last example is a negative number. The scientific notation version will thus be -4.4 x 10?, Let's determine the exponent:

That means moving the decimal five places to the right:

to get our scientific notation,
![]()
Practice problems
Convert these numbers to scientific notation form.
| 1. 0.00154 | |
| 2. 105060 | |
| 3. -0.00221 |
| 4. -125,000 | |
| 5. -10.23 | |
| 6. 1,525,000,000 |
| 7. 0.198 | |
| 8. -0.01023 | |
| 9. 6022000000 |
Multiplication of numbers in scientific notation
Multiplication of numbers in scientific notation is easy. That's in large part why we even use it. We make use of the commutative property of multiplication, that is a·b = b·a, and a·b·c = a·c·b, and so on.
Example 5
$(2.1 \times 10^{11})(3.2 \times 10^3)$
There are two keys to doing this kind of multiplication. The first is that the only operation there is multiplication, so we can easily rearrange this expression to
$$(2.1)(3.2)(10^11)(10^3)$$
Now the first multiplication is just 2.1(3.2) = 6.7 (I'm being careful not to use more digits after the decimal than I should). The second key is that when we multiply powers of the same base, we just add exponents. It's pretty easy to see why with a simple example like (103)(102):
$$ \begin{align} (10^3)(10^2) &= (10 \cdot 10\cdot 10)(10\cdot 10) \\[5pt] &= 10 \cdot 10 \cdot 10 \cdot 10 \cdot 10 \\[5pt] &= 10^5 \\ &= 10^{3 + 2} \end{align}$$
That makes our example
$$6.7 (10^{11 + 3}) = 6.7 \times 10^{14}$$
Example 6
$(3.21 \times 10^{{-12})(9.09 \times 10^4)$
In this example, $3.21 \times 9.09 = 29.2$ We don't like to have two digits before the decimal in scientific notation, so we're going to have to adjust the exponent of our result at the end to put it back to the proper place. First we rearrange to
$$= (3.21)(9.09)(10^{-12})(10^4)$$
Then multiplying the numbers and powers of ten separately we have
$$= 29.2 \times 10^{-12 + 4} = 29.2 \times 10^{-8}$$
Now we've got to move the decimal one place to the left to get this number into proper form. Notice that the "instruction" $10^{-8}$ says to move the decimal 8 places to the left. If we do one of these moves ahead of time, we'll have to change that "instruction" to $10^{-7}$, so our result is
$$2.92 \times 10^{-7}$$
Take a minute to convince yourself that $29.2 \times 10^{-8}$ and $2.92 \times 10^{-7}$ are the same number.
Practice problems
Multiply these numbers and express the results in proper scientific notation:
-
$(1.0 \times 10^7)(2.1 \times 10^3)$
Solution
$$ \begin{align} &= 1.0 \cdot 2.1 \cdot 10^7 \cdot 10^3 \\[5pt] &= 2.1 \times 10^{7+3} \\[5pt] &= 2.1 \times 10^{10} \end{align}$$
-
$(9.2 \times 10^6)(4.3 \times 10^2)$
Solution
$$ \begin{align} &= 9.2 \cdot 4.3 \cdot 10^6 \cdot 10^2 \\[5pt] &= 39.56 \times 10^{6+2} \\[5pt] &= 39.56 \times 10^{8} \\[5pt] &= 3.956 \times 10^9 \end{align}$$
-
$(6.26 \times 10^{-3})(3.21 \times 10^{14})$
Solution
$$ \begin{align} &= 6.26 \cdot 3.21 \cdot 10^{-3} \cdot 10^{14} \\[5pt] &= 20.1 \times 10^{14-3} \\[5pt] &= 20.1 \times 10^{11} \\[5pt] &= 2.01 \times 10^{10} \end{align}$$
-
$6.022 \times 10^{23})(1.981 \times 10^{-19})$
Solution
$$ \begin{align} &= 6.022 \cdot 1.981 \cdot 10^{23} \cdot 10^{-19} \\[5pt] &= 11.93 \times 10^{23-19} \\[5pt] &= 11.93 \times 10^{4} \\[5pt] &= 1.193 \times 10^{5} \end{align}$$
-
$(6.022 \times 10^{23})(2 \times 10^2)$
Solution
$$ \begin{align} &= 6.022 \cdot 2 \cdot 10^{23} \cdot 10^{2} \\[5pt] &= 12.044 \times 10^{23+2} \\[5pt] &= 12.044 \times 10^{25} \\[5pt] &= 1.204 \times 10^{26} \end{align}$$
-
$(3.11 \times 10^{18})(3.11 \times 10^{-19})$
Solution
$$ \begin{align} &= 3.11^2 \cdot 10^{18} \cdot 10^{-19} \\[5pt] &= 9.76 \times 10^{18-19} \\[5pt] &= 9.76 \times 10^{-1} \\[5pt] &= 0.976 \end{align}$$
(Scientific notation isn't always necessary. We use it to display and manipulate cumbersome numbers, but 0.976 is a "reasonable" number.
-
$(4.2822 \times 10^3)(4.2822 \times 10^{-5})$
Solution
$$ \begin{align} &= 4.2822^2 \cdot 10^{3} \cdot 10^{-5} \\[5pt] &= 18.34 \times 10^{3-5} \\[5pt] &= 18.34 \times 10^{-2} \\[5pt] &= 0.1834 \times 10^{5} \end{align}$$
(Scientific notation isn't always necessary. We use it to display and manipulate cumbersome numbers, but 0.1834 is a "reasonable" number.
-
$(2.22 \times 10^{-4})(2.22 \times 10^{-11})$
Solution
$$ \begin{align} &= 2.22^2 \cdot 10^{-4} \cdot 10^{-11} \\[5pt] &= 4.93 \times 10^{-4-11} \\[5pt] &= 4.93 \times 10^{-15} \end{align}$$
-
$(0.334)(7.1 \times 10^{-10})$
Solution
$$ \begin{align} &= 0.344 \cdot 7.1 \times 10^{-10} \\[5pt] &= 2.37 \times 10^{-10} \end{align}$$
-
$(101,325)(1.981 \times 10^{-19})$
Solution
$$ \begin{align} &= 101,325 \cdot 1.981 \cdot 10^{-19} \\[5pt] &= 200,724 \times 10^{-19} \\[5pt] &= 2.007 \times 10^{-14} \end{align}$$
Division of numbers in scientific notation
Division of one number in scientific notation by another is as straightforward as multiplication. Divide the numbers, then divide the powers of 10 and multiply the two. Remember that when we divide powers of the same base, we subtract the exponent of the denominator from the exponent of the numerator. Here's an example:
$$ \require{cancel} \frac{10^3}{10^2} = \frac{\cancel{10} \cdot \cancel{10} \cdot 10}{\cancel{10} \cdot \cancel{10}} = 10^{3-2} = 10^1$$
Here's an example of a division problem in scientific notation. We can regroup the decimal numbers and powers of ten:
$$\frac{4.22 \times 10^{14}}{2.31 \times 10^{10}} = \frac{4.22}{2.31} \cdot \frac{10^{14}}{10^{10}}$$
Then it's just a matter of dividing 4.22 by 2.31 and subtracting the exponents of the powers of ten to get the result.

Here are some practice exercises ...
Practice problems
Divide these numbers and express the results in proper scientific notation:
-
$$\frac{2.1 \times 10^4}{1.1 \times 10^2}$$
Solution
$$ \begin{align} &= \frac{2.1}{1.1} \times \frac{10^4}{10^2} \\[5pt] &= 1.92 \times 10^{4-2} \\[5pt] &= 1.91 \times 10^2 = 191 \end{align}$$
(Scientific notation isn't always necessary. We use it to display and manipulate cumbersome numbers, but 191 is a "reasonable" number.
-
$$\frac{1.91 \times 10^{8}}{2.25 \times 10^{11}}$$
Solution
$$ \begin{align} &= \frac{1.91}{2.25} \times \frac{10^8}{10^{11}} \\[5pt] &= 0.849 \times 10^{8-11} \\[5pt] &= 0.849 \times 10^{-3} \\[5pt] &= 8.49 \times 10^{-4} \end{align}$$
-
$$\frac{6.022 \times 10^{23}}{1.55 \times 10^{18}}$$
Solution
$$ \begin{align} &= \frac{6.022}{1.55} \times \frac{10^{23}}{10^{18}} \\[5pt] &= 3.88 \times 10^{23-18} \\[5pt] &= 3.88 \times 10^{5} \end{align}$$
-
$$\frac{2.381 \times 10^{-9}}{6.8 \times 10^{-6}}$$
Solution
$$ \begin{align} &= \frac{2.381}{6.8} \times \frac{10^{-9}}{10^{-6}} \\[5pt] &= 0.350 \times 10^{-9-(-6)} \\[5pt] &= 0.350 \times 10^{-3} \\[5pt] &= 3.50 \times 10^{-4} \end{align}$$
-
$$\frac{1 \times 10^{-15}}{2 \times 10^2}$$
Solution
$$ \begin{align} &= \frac{1}{2} \times \frac{10^{-15}}{10^{2}} \\[5pt] &= 0.5 \times 10^{-15-2} \\[5pt] &= 0.5 \times 10^{-17} \\[5pt] &= 5.0 \times 10^{-18} \end{align}$$
-
$$\frac{5.749 \times 10^6}{2.11 \times 10^{11}}$$
Solution
$$ \begin{align} &= \frac{5.749}{2.11} \times \frac{10^6}{10^{11}} \\[5pt] &= 2.79 \times 10^{6-11} \\[5pt] &= 2.79 \times 10^{-5} \end{align}$$
Scientific notation on a calculator or computer: EE

On most calculators, we enter scientific notation using a button labeled EXP or EE. Both are a shorthand for "
For example, if I want to enter 2.48 x 1019, on the calculator on the left, I enter the sequence "2.48
Four calculations – two multiplications and two divisions – have been entered and performed on this calculator. The first multiplication is
(2.48 x 1019)(5.81 x 10-6)
and the result is
1.44 x 1014.
See if you can follow the rest of those calculations.
You can set your calculator to display results in scientific notation if you'd like. On this calculator it's done using the [MODE] button.
Notice that the 3rd result was displayed as 4613.333. This calculator was not set up to use scientific notation for numbers in the 1000 range.
Some calculators have a mode such that when you press something like EE 4, it will print 104. That's nice, but not completely necessary. Use it if you have it and you like it.
Historical note: EE stands for Engineering Exponent. It was a calculator shortcut developed at a time when most calculator users were engineers.

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2016-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.