Follow the electrons
The concept of oxidation number or oxidation state can be very useful for understanding what's going on in a reaction beneath the balanced equation. In another section we'll deal with reduction-oxidation ("redox") reactions, but in this section, we'll assign oxidation numbers to atoms and use them to determine whether or not the reaction is a redox reaction, and which atoms are oxidized and reduced in it.
Remember that oxidation is a loss of electrons and reduction is a gain of electrons. I know, it doesn't seem very logical for "reduction" to mean "gain." The term "oxidation" is an old one and refers to a time when what we now refer to as oxidation was only observed to occur in the presence of oxygen. At the time we didn't really understand the part about transfer of electrons. Like a lot of terminology in science, we're stuck with oxidation and reduction.
Oxidation & reduction
Oxidation is a loss of electron(s) and Reduction is a gain of electron(s)
Oxidation numbers
Oxidation numbers are made-up or hypothetical numbers assigned to each atom in a reaction, individual or within a molecule. They represent, loosely, the number of electrons available for shuffling around during the course of a reaction. Reactions can proceed with or without the exchange of electrons. If electrons are exchanged, that will be reflected in the difference in oxidation number of atoms on the right and left side of the chemical equation, and the reaction is called a redox (reduction-oxidation) reaction.
There are agreed-upon rules for assigning oxidation numbers. I'll go through them here, but they're recapped in the table below. You can click on the table to download a .pdf copy of it.
The rules
- Atoms in elemental form have an oxidation number of zero. For example, Mg, H2, Ar and Fe(s) are all examples of atoms in their elemental states. In the case of Mg, if no charge and no state are shown, we have to assume it's metallic Mg. Hydrogen exists as a diatomic gas in its elemental form.
- Group 1A and 2A elements have the same oxidation number that the ion of the metal would, unless it's in its elemental form.
- Hydrogen almost always has an oxidation number of $+1$. A rarely-encountered exception is when H is bound to a metal in a hydride compound.
- Oxygen almost always has an oxidation number of $-2$. In rare exceptions, when oxygen is in a peroxide (O2-, like H2O2), its oxidation number is $-1$.
- Fluorine always has a $-1$ oxidation number, and Cl, Br and I almost always do.
This may be the most important rule: The oxidation numbers of a molecule have to add up to the total charge on the molecule. If the molecule is neutral, that's zero. For example, the sums of the oxidation numbers of CO2 and CO32- are $0$ and $-2$, respectively.
Rules for assigning oxidation numbers
| Substance | Ox. no. |
Notes |
|---|---|---|
| Elemental form | 0 | Only one kind of atom is present, no charge. |
| Atomic ions | Takes the charge of the atom (monatomic ion) | |
| Group 1A: Li, Na, K, Rb, Cs | +1 | Unless in elemental form |
| Group 2A: Be, Mg, Ca, Sr, Ba | +2 | Unless in elemental form |
| Hydrogen (H) | +1 | When bonded to a nonmetal, -1 when bonded to a metal |
| Oxygen (O) | -1 | in peroxides, O2- |
| Oxygen (O) | -2 | in all other oxygen compounds |
| Fluorine (F) | -1 | always |
| Neutral compounds | * | *The sum of all oxidation numbers of atoms or ions in a neutral compound is zero, always |
You've noticed that some oxidation numbers are fixed, and others can vary (otherwise we wouldn't have redox reactions). It turns out that the oxidation numbers of some atoms can vary quite a lot.
The chart below should help you to visualize the possible oxidation numbers that can occur for the first 39 atoms.
If you're working out the oxidation states of the atoms in a reaction and you get one that's not on this chart, it's probably worth checking your work. You can download the table above and the chart below here:
Below you'll find a few examples of how we use oxidation numbers to make some judgments about chemical reactions. The general approach is to assign oxidation numbers to each atom (remember, its each atom, not molecule),
then compare the oxidation number of any given atom on both the left and right sides of the reaction. If the oxidation state (number) of the atom increases, that atom is oxidized (loses electrons). If the oxidation state decreases from left to right, that atom is reduced.
If the oxidation number of an atom increases from left to right in a reaction, the atom is oxidized in the process. If it decreases, the atom is reduced.
Practice problems
Assign oxidation numbers to each atom in the molecule.
-
$Al_2O_3$
Solution
$$Al: \; +3 \phantom{0000} O: \; -2$$
-
$XeF_6$
Solution
$$Xe: \; +6 \phantom{0000} F: \; -1$$
-
$K_2Cr_2O_7$
Solution
$$K: \; +1 \phantom{0000} Cr: +6\; \phantom{0000} O: \; -2$$
-
$Ca(ClO)_2$
Solution
$$Ca: \; +6 \phantom{0000} Cl: \; -1 \phantom{0000} O: \; -2$$
-
$Na_2SO_3$
Solution
$$Na: \; +4 \phantom{0000} S: \; -2 \phantom{0000} O: \; -2$$
-
$Fe(NO_3)_3$
Solution
$$Fe: +3\; \phantom{0000} N: +5 \; \phantom{0000} O: \; -2$$
Example 1
In order to tell if a reaction is a reduction-oxidation (redox) reaction, we assign oxidation numbers to each atom. By the way, the reaction doesn't need to be balanced for this oxidation-number analysis, but this one is.
In this reaction, the Mg is presumed to be in its metallic elemental form because it doesn't have a charge, so its oxidation number is zero. Hydrogen bound to a nonmetal has a $+1$ ox. number, and the Cl gets a $-1$ because the sum of oxidation numbers of a neutral compound has to be zero.
Mg is a group 2A element, so it has a $+2$ oxidation number, and the hydrogens of H2 have zero ox. numbers because that's hydrogen in its elemental form.

Now let's look at the results. Mg goes from an oxidation state of $0$ to $+2$. It's oxidation number increases, so Mg is oxidized in this reaction and we refer to HCl as an oxidizing agent or an oxidizer. Likewise, the oxidation number of H is reduced from $+1$ to zero, so hydrogen is reduced in this reaction and Mg is referred to as a reducing agent.

Example 2
This is an acid-base neutralization reaction. Here are the assignments of the oxidation numbers; they're pretty straightforward:

But this time when we compare oxidation numbers on the right and left, nothing has changed. No electrons have been transferred, so this is not a redox reaction.

Example 3
In this example, we have two cases where we assign a -2 oxidation number to oxygen, and we are left to assign the oxidation number of the nitrogen so that the sum of all of the ox. numbers is zero because these are neutral molecules.

Comparing left and right sides, we see that the sulfur is oxidized; it's ox. number goes from -2 to zero. And the nitrogen is reduced (+4 → +2). This is a redox reaction.

In an oxidation reaction, the chemical component (sometimes referred to generically as a "species") that is reduced is also called an oxidizing agent or an oxidizer because its presence oxidizes another component. In this reaction, nitric acid (HNO3) is an oxidizer and H2S is a reducing agent (note that we don't usually call it a "reducer").
In a redox reaction, the species that is oxidized is also called a reducing agent, and the species that is reduced is also called an oxidizing agent or just an oxidizer.
Example 4
Here's another example in which we have to assign an oxidation number based only on the fact that the sum of oxidation numbers of neutral compounds has to be zero (the +4 on the C of CaCO3). The resulting analysis shows that this is not a redox reaction.

Notice that the analysis of oxidation states in a chemical equation does not depend on the numerical coefficients (number of moles) of any reactant or product.

Practice problems
For each reaction below, (1) balance the reaction if necessary, (2) assign oxidation numbers to each atom, (3) identify which atoms are oxidized and reduced, if any, and (4) identify the oxidizing and reducing agents, if any.
-
$CuO + Zn \longrightarrow ZnO + Cu$
Solution

-
$2 \, H_2S + SO_2 \longrightarrow 3 \, S + 2 \, H_2O$
Solution

-
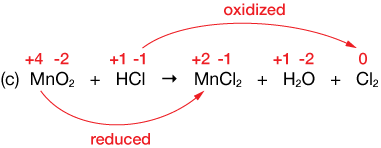
$MnO_2 + HCl \longrightarrow MnCl_2 + H_2O + Cl_2$
Solution
-
$KMnO_4 + HCl \longrightarrow KCl_2 + MnCl_2 + H_2O + Cl_2$
Solution

-
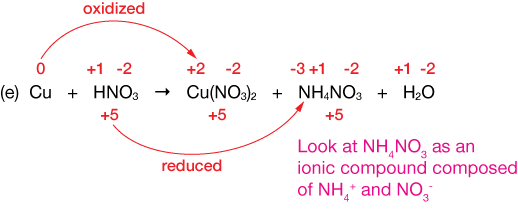
$Cu + HNO_3 \longrightarrow Cu(NO_3)_2 + NH_4NO_3 + H_2O$
Solution
-
$H_2SO_4 + HBr \longrightarrow SO_2 + Br_2 + H_2O$
Solution


![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2019-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.
