What is stoichiometry?
When the space shuttle took off it used two different rocket systems. The main engines were powered by combining oxygen and hydrogen in the highly exothermic reaction
$$2 \, H_2 + O_2 \longrightarrow 2 \, H_2O$$
The fuel was stored in that brown tank in liquid form as liquid oxygen (LOX) and liquid hydrogen (LH2). Liquids are much more dense than gases, so storage in the liquid phase allows the shuttle to carry an immense amount of fuel.
Unfortunately, that fuel made up the majority of the weight of the shuttle at liftoff. So most of what the shuttle engines lifted from the ground was fuel. That means it was very important not to have too much of one or the other reactant on board. We'd want just enough hydrogen to react with the amount of oxygen on board, and vice versa. That's where stoichiometry and moles are essential.
And maybe you can be the one to invent a kind of rocket propulsion that will release more energy per pound of fuel!

Stoichiometry is the relationship between the relative quantities of substances involved in a chemical reaction, typically ratios of whole numbers. Stoichiometry is a catch all word for all of the calculations we do to determine how much of what to mix with what.
Example 1 – Space shuttle fuel mixture
Let's begin with a calculation relevant to our opening example: The space shuttle LOX tank held 629,340 Kg (about 630 metric tons) of LOX. How many kilograms of Hydrogen would it need to carry in order to assure that the reaction
$$2 \, H_2 + O_2 \longrightarrow 2 \, H_2O$$
goes to completion, with no reactants remaining? (The backward rate of this reaction is extremely slow compared to the explosive forward rate, thus the single arrow)
$$ \begin{align} \require{cancel} 629,340 \; \cancel{Kg \; O_2} &\left( \frac{1000 \; \cancel{g \; O_2}}{1 \; \cancel{Kg \; O_2}} \right)\left( \frac{1 \; mol \; O_2}{32 \; \cancel{g \; O_2}} \right) \\[5pt] &= 1.97 \times 10^7 \; mol \; O_2 \end{align}$$
In the calculation above, I've combined the conversion from Kg to grams with the calculation of the number of moles of O2 (32 g/mol). That's easy if we keep track of units. Now that we have the number of moles of O2, we use the balanced chemical equation (that's what it's for!) to find out how many moles of H2 we need. From the coefficients, you can see that two moles of H2 are consumed for every one mole of O2, so we need 3.94 x 107 mol of H2 (1.97 x 2 = 3.94)
The mole ratio told us that in this reaction exactly twice the number of moles of H2 are used as O2. Now it's just a simple matter of converting 3.94 x 104 mol of H2 to Kg of H2.
We could move forward and complete the calculation one step at a time - moles O2 to moles H2 to grams H2 to Kg H2, but there's a faster way if we make good use of units and cancellation of units. Here's how to combine the second part of the calculation into one big step:
$$1.97 \times 10^7 \; \cancel{mol \; O_2} \left( \frac{2 \cancel{mol \; H_2}}{1 \; \cancel{mol \; O_2}} \right) \left( \frac{2 \; \cancel{g \; H_2}}{1 \; \cancel{mol \; H_2}} \right) \left( \frac{1 \; Kg \; H_2}{1000 \; \cancel{g \; H_2}} \right) = 7.9 \times 10^4 \; Kg \; H_2$$
The first parentheses is the mole ratio: two H2 for every one O2. The second is the formula weight of H2, and the third converts from grams to Kilograms.
Notice that in each of the large fractions above, a relationship and its units are written, and written in such a way that the units cancel with those of the previous term to get us closer to the desired units, in this case Kg of H2.
So about 79,000 Kg of H2 would be needed to react with 629,340 Kg of LOX.
It turns out that in practice the shuttles carried a little more LH2 than that because of engine efficiency issues and because the rate of loss of lighter LH2 due to evaporation during the wait for launch is higher than that of LOX.
Nevertheless, I hope you get the idea. This is a very important calculation and it depends 100% on doing good stoichiometry.
Example 2
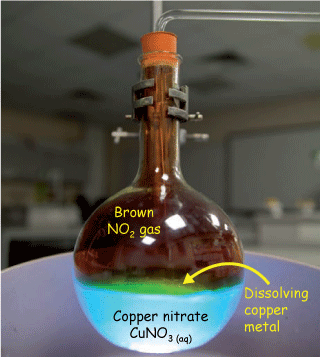
How many moles of nitric acid (HNO3) are required to completely dissolve 1 g of copper (Cu) according to the reaction
$$2 \, HNO_{3 \; (aq)}+Cu_{(s)} \rightleftharpoons CuNO_{3 \; (aq)}+NO_{2 \; (g)}+H_2O_{(l)}$$
$$1 \; \cancel{g \; Cu} \left( \frac{1 \; mol \; Cu}{63.54 \; \cancel{g \; Cu}} \right) = 1.57 \times 10^{-2} \; mol \; Cu$$
Now that we've got the number of moles of copper, the balanced equation (check it!) tells us that for every one mole of Cu, 2 moles of nitric acid are needed, so we need 3.64 x 10-2 moles of HNO3. That's it!
We could, of course, convert the number of moles of HNO3 to grams and then even to milliliters of HNO3 solution if we know its concentration.
Example 3
In the above example, how many grams of a 30% (by weight) aqueous solution of HNO3 would be required to run the reaction to completion?
$$ \begin{align} 3.64 \times 10^{-2} \; \cancel{mol \; HNO_3} &\left( \frac{63 \; g \; HNO_3}{1 \; \cancel{mol \; HNO_3}} \right) \\[5pt] &= 2.29 \; g \; HNO_3 \end{align}$$
That's how many grams of HNO3 we need, now we need to find out how much of the 30% solution that translates to.The calculation we want is:
$$ \begin{align} 2.29 \; \cancel{g \; HNO_3} &\left( \frac{100 \; g \text{ solution}}{30 \; \cancel{g \; HNO_3}} \right) \\[5pt] &= 7.6 \; g \text{ of 30% solution} \end{align}$$
Often (particularly in the biological sciences) solution concentrations are given in percent, either weight of solute divided by total weight of solution %(w/w) or weight of solute over total volume of solution %(w/v).
See the notes on concentration to brush up on how to specify the concentration of solutions.
Example 4
n-octane (C8H18) is a principal component of gasoline. It oxidizes (burns) in the presence of oxygen according to the equation
$$2 \, C_8H_{18 \; (g)}+25 \, O_{2 \;(g)} \rightleftharpoons 16 \, CO_{2 \; (g)} + 18 \, H_2O_{(g)}$$
If 1 gallon of gasoline is burned in the presence of excess oxygen (i.e. enough oxygen to make the reaction go to completion), how many liters of CO2 gas are emitted into the atmosphere? There are 3.785 liters in a gallon and the density of gasoline is about 0.75 Kg/liter. At atmospheric temperature and pressure, 1 mole of CO2 gas takes up about 22.4 liters of volume.

Now we can use the density of gasoline to find the approximate mass of octane (we're assuming that gas is 100% octane here, not too bad an approximation).
Here's the mass of octane:
$$ \begin{align} 3.785 \; \cancel{\text{liters }C_8H_{18}} &\left( \frac{750 \; g \; C_8H_{18}}{1 \; \cancel{\text{liter }C_8H_{18}}} \right) \\[5pt] &= 2.838 \times 10^3 \; g \; C_8H_{18} \end{align}$$
Now convert to moles:
$$ \begin{align} 2.838 \times 10^3 \; \cancel{g \; C_8H_{18}} &\left( \frac{1 \; mol C_8H_{18}}{114 \; \cancel{g \; C_8H_{18}}} \right) \\[5pt] &= 24.9 \; mol \; C_8H_{18} \end{align}$$
Now we use the mole ratio in the balanced equation: For every two moles of C8H18 we form 16 moles of CO2.
$$ \begin{align} 24.9 \; \cancel{mol \; C_8H_{18}} &\left( \frac{16 \; mol CO_2}{2 \; \cancel{mol \; C_8H_{18}}} \right) \\[5pt] &= 199 \; mol \; CO_2 \end{align}$$
Now we can use the molar volume of CO2 gas (the volume that one mole takes up - given) to find our result:
$$ \begin{align} 199 \; \cancel{mol \; CO_2} &\left( \frac{22.4 \; L \; CO_2}{1 \; \cancel{mol \; CO_2}} \right) \\[5pt] &= 4458 \text{ liters of }CO_2 \end{align}$$
There are 1000 liters in a cubic meter, so that's about 4.5 cubic meters of CO2 gas emitted from every gallon of gas burnt. Something to think about.
Now we could easily have done this whole calculation at once by just multiplying the many terms in parenthesis above and canceling units. Here's how it looks:
$$1 \; \cancel{gal \; C_8H_{18}} \left( \frac{3.785 \; \cancel{L}}{1 \cancel{gal}} \right)\left( \frac{750 \; \cancel{g \; C_8H_{18}}}{1 \; \cancel{L C_8H_{18}}} \right) \left( \frac{1 \; \cancel{mol \; C_8H_18}}{114 \; \cancel{g \; C_8H_{18}}} \right) \left( \frac{16 \; \cancel{mol \; CO_2}}{2 \; \cancel{mol \; C_8H_{18}}} \right) \left( \frac{22.4 \; L \; CO_2}{1 \; \cancel{mol \; CO_2}} \right)$$
All of the units cancel nicely in a nice progression from gallons of octane to liters of CO2. Remember that division is just multiplication by the reciprocal and that multiplication is commutative, so these numbers can be multiplied and divided on a calculator in any order, like:
= 3.785 · 750 · 16 · 22.4 / (114 ·2) liters
PS: When you study gases, you'll learn that a mole of any gas at standard temperature and pressure, T = 273K and P = 1 atm, occupies 22.4 liters. That was Avogadro's original conjecture.
Example 5 — Limiting reactant
Gold (Au) metal reacts with chlorine gas (Cl2) to form gold (III) chloride, AuCl3.
$$2 \, Au_{(s)} + 3 \, Cl_{2 \; (g)} \rightleftharpoons 2 \, AuCl_{3 \; (g)}$$
How many grams of AuCl3 will be formed if 1 g of gold metal is reacted with 2 g of Cl2 ?
That means that one of the reactants will run out first, and we call this the limiting reactant. When it runs out, the reaction has to stop. So our first job in a problem like this is to find out which reactant is limiting.
We start by calculating the number of moles of each reactant present:
$$ \begin{align} 1.0 \; \cancel{g \; Au} \left( \frac{1 \; mol \; Au}{197 \; \cancel{g \; Au}} \right) &= 0.005 \; mol \; Au \\[5pt] g.0 \; \cancel{g \; Cl_2} \left( \frac{1 \; mol \; Cl_2}{70.97 \; \cancel{g \; Cl_2}} \right) &= 0.028 \; mol \; Cl_2 \end{align}$$
Now look again at the reaction:
$$2 \, Au_{(s)} + 3 \, Cl_{2 \; (g)} \rightleftharpoons 2 \, AuCl_{3 \; (g)}$$
It says that for every 2 moles of gold, we need 3 moles of chlorine gas. In fact, it's easy to see that we have more Cl2 than that. Look at it the other way: The equation says that for every three moles of Cl2, we need 2 moles of gold. It's not even close. Either way we look at it, Au is the limiting reactant.
Now that we've identified the limiting reactant, the trick is to base all further calculations on it, because its amount alone determines how far the reaction will proceed. Now it's just a matter of finding out how many moles of AuCl3 will be formed:
$$ \begin{align} 0.005 \; \cancel{mol \; Au} &\left( \frac{2 \; mol \; AuCl_3}{2 \; \cancel{mol \; Au}} \right) \\[5pt] &= 0.005 \; mol \; AuCl_3 \end{align}$$
(We could have done that just by inspection, of course!). Finally, the mass of AuCl3 produced:
$$ \begin{align} 0.005 \; \cancel{mol \; AuCl_3} &\left( \frac{303 \; g \; AuCl_3}{1 \; \cancel{mol \; AuCl_3}} \right) \\[5pt] &= 1.52 \; g \; AuCl_3 \end{align}$$
Example 6 — Limiting reactant
152 grams of carbon monoxide (CO) are added to 24 g of hydrogen gas (H2) to produce methanol (CH3OH). How many grams of methanol* will be produced?
*
$$CO + H_2 \longrightarrow CH_3OH$$
Well, that's an unbalanced reaction, so we ought to balance it. That turns out to be simple:
$$CO + 2 \, H_2 \longrightarrow CH_3OH$$
Now we just need to calculate the number of moles of CH3OH we could expect if 24 g of H2 reacted completely (assuming excess CO) and if 152 g of CO reacted completely (assuming excess H2). The lesser number of moles will be the theoretical maximum number of moles of CH3OH formed. Here are the calculations:
$$ \begin{align} 24 \; \cancel{g \; J_2} &\left( \frac{1 \; mol \; H_2}{2 \; \cancel{g \; H_2}} \right)\left( \frac{1 \; mol\; CH_3OH}{2 \; \cancel{mol \; H_2}} \right) \\[5pt] &=6 \; mol \; CH_3OH \\[5pt] 152 \; \cancel{g \; CO} &\left( \frac{1 \; mol \; CO}{28 \; \cancel{g \; CO}} \right)\left( \frac{1 \; mol \; CH_3OH}{1 \; \cancel{mol \; CO}} \right) \\[5pt] &= 5.42 \; mol \; CH_3OH \end{align}$$
The CO is the limiting reactant because its amount will produce the least CH3OH in this scenario.
Now we just need to calculate the mass of CH3OH that is 5.4 moles:
$$ \begin{align} 5.4 \; \cancel{mol \; CH_3OH} &\left( \frac{32 \; g \; CH_3OH}{1 \; \cancel{mol \; CH_3OH}} \right) \\[5pt] &= 173 \; g \; CH_3OH \end{align}$$
There will be excess hydrogen gas (H2) left over after all of the CO is exhausted. You can easily calculate the number of moles and the mass of H2 that will be.
Solving limiting reactant problems
To solve a limiting reactant problem, calculate the number of moles of the desired product that would be obtained from each of the reactants, assuming an excess of all other reactants. The reactant that yields the least product is the limiting reactant, and that yield of product is the theoretical maximum yield of the reaction.
Example 7 — One more limiting reactant problem ...
93 Kg of nitrogen gas (N2) are added to 265.8 Kg of hydrogen gas (H2) to produce ammonia gas (NH3). How much ammonia (in Kg) can be produced by this reaction?
$$N_2 + 3\, H_2 \rightleftharpoons 2 \, NH_3$$
Now we calculate the number of moles of NH3 we could expect if 93 Kg of N2 reacted completely (assuming excess H2) and if 265.8 Kg of H2 reacted completely (assuming excess N2). The lesser number of moles will be the theoretical maximum number of moles of NH3 formed. First the N2:
$$ \begin{align} 93,333 \; \cancel{g \; N_2} &\left( \frac{1 \; \cancel{mol \; N_2}}{28 \; \cancel{g \; N_2}} \right) \left( \frac{2 \; mol \; NH_3}{1 \; \cancel{mol \; N_2}} \right) \\[5pt] &= 6,643 \; mol \; NH_3 \end{align}$$
Then the H2:
$$ \begin{align} 265,800 \; \cancel{g \; H_2} &\left( \frac{1 \; \cancel{mol \; H_2}}{2 \; \cancel{g \; H_2}} \right) \left( \frac{2 \; mol \; NH_3}{3 \; \cancel{mol \; N_2}} \right) \\[5pt] &= 5.9 \times 10^8 \; mol \; NH_3 \end{align}$$
Well, it's very easy to see that the limiting reagent is N2. When 6,643 moles of ammonia has been produced, the supply of N2 will be exhausted, with many more moles of H2 remaining. Now we just need to calculate the mass of NH3 that constitutes 6,643 moles:
$$6,643 \; \cancel{mol \; NH_3} \left( \frac{17 \; g \; NH_3}{1 \; \cancel{mol \; NH_3}} \right) = 112.9 \; Kg \; NH_3$$
Why is the synthesis of ammonia (NH3) important?

You will recall from your study of bonding that the triple bond in N-N is very strong. In fact, there are only a couple of things in nature that can break it. One is an energetic electric arc like in lightning. Another very important one is the biochemistry of plants called legumes. These plants can break the N-N bond to form atomic nitrogen thats available for use in making amino acids and nucleic acids. We couldn't get along without them.
In modern agriculture, it's very important to have non-N2 nitrogen available in the soil, but that supply gets depleted over time as we grow more food on the land, so we need to add nitrogen-rich fertilizer to it. The primary source of that nitrogen is fertilizer. The reaction to synthesize ammonia is run using a catalyst and the process is called the Haber process. It's one of the most important chemical reactions we have.
Practice problems
$\longleftarrow$ To view wide equations in solutions, scroll left | right. $\longrightarrow$
-
Consider the reaction
$N_{2 \; (g)} + F_{2 \; (g)} \longrightarrow NF_{3 \; (g)}$
Balance the equation if necessary. If 27.5 g of nitrogen gas (N2) are used in the reaction, what mass of fluorine (F2) would be needed for the reaction to go to completion?
Solution
The balanced equation is: $N_2 + 3 \, F_2 \longrightarrow 2 \, NF_3$.
$$27.5 \; \cancel{g \; N_2} \left( \frac{1 \; \cancel{mol \; N_2}}{28 \; \cancel{g \; N_2}} \right) \left( \frac{3 \; \cancel{mol \; F_2}}{1 \; \cancel{mol \; N_2}} \right) \left( \frac{38 \; g \; F_2}{1 \; \cancel{mol \; F_2}} \right) = \frac{27.5 \cdot 3 \cdot 38}{28} = 112 \; g \; F_2$$
The formula weight of N2 is 28 g/mol (2 × 14, the atomic weight of nitrogen) and the FW of F2 is 38 g/mol (2 × 19, the atomic weight of fluorine).
-
Consider the reaction
$C_6H_{10 \; (g)} + O_{2 \; (g)} \longrightarrow CO_{2 \; (g)} + H_2O_{(g)}$
Balance the equation if necessary. If only 18.3 g of O2 is available, how much C6H10 (cyclohexene) can be reacted? How much CO2 (in grams) would be produced by this reaction?
Solution
The balanced equation is: $2 \, C_6H_{10} + 17 \, O_2 \longrightarrow 12 \, CO_2 + 10 \, H_2O$
$$ \begin{align} 18.3 \; \cancel{g \; O_2} \left( \frac{1 \; \cancel{mol \; O_2}}{32 \; \cancel{g \; O_2}} \right) \left( \frac{12 \; \cancel{mol \; CO_2}}{17 \; \cancel{mol \; O_2}} \right) \left( \frac{44 \; g \; CO_2}{1 \; \cancel{mol \; CO_2}} \right) &= \frac{18.3 \cdot 12 \cdot 44}{32 \cdot 17} \\[5pt] &= 17.8 \; g \; CO_2 \end{align}$$
This is a combustion reaction.
-
Consider the reaction
$HBr_{(aq)} + KHCO_{3 \; (aq)} \longrightarrow H_2O_{(l)} + KBr_{(aq)} + CO_{2 \; (g)}$
- Balance the reaction. What type of reaction is this?
- How many moles of KBr would be formed by reacting 3.3 moles of HBr with plenty of KHCO3?
- How many grams of KBr would be formed by reacting 3.3 moles of HBr with only 1.2 moles of KHCO3?
Solution
(a) The equation is balanced. (b) ↓
$$3.33 \; \cancel{mol \; HBr} \left( \frac{1 \; mol \; KBr}{1 \; \cancel{mol \; HBr}} \right) = 3.3 \; mol \; KBr$$
(c) In this reaction HBr and KHCO3 are in a 1:1 ratio, so 1.2 moles of KHCO3 is the limiting reactant:
$$1.2 \; \cancel{mol \; KHCO_3} \left( \frac{1 \; \cancel{mol \; KBr}}{1 \; \cancel{mol \; KHCO_3}} \right) \left( \frac{119 \; g \; KBr}{1 \; \cancel{mol \; Kbr}} \right) = 143 \; g \; KBr$$
N.B. This reaction is difficult to classify. It's part displacement and part decomposition (more products than reactants). Many reactions don't fit neatly into any one category.
-
In 2018, citizens and businesses of the US burned about 29.9 trillion cubic feet of natural gas. That's roughly 1.6 × 109 Kg of propane, if we assume that natural gas is mostly propane (not a terrible assumption, but it also contains methane, butane and other small hydrocarbon gases and contaminants).
- Write a balanced reaction for the combustion of propane, C3H8.
- Assuming plenty of oxygen is available for the reactions, calculate the number of metric tons (1 metric ton = 1000 Kg) of CO2 that are released into the atmosphere by these reactions.
Solution
The balanced equation is
$$C_3H_8 + 5 \, O_2 \longrightarrow 3 \, CO_2 + 4 \, H_2O$$
$$ \begin{align} 1.6 \times 10^9 \; \cancel{Kg \; C_3H_8} &\left( \frac{1 \; \cancel{mol \; C_3H_8}}{0.044 \; \cancel{Kg \; C_3H_8}} \right) \left( \frac{3 \; \cancel{mol \; CO_2}}{1 \; \cancel{mol \; C_3H_8}} \right) \left( \frac{0.044 \; Kg \; CO_2}{1 \; \cancel{mol \; CO_2}} \right) \\[5pt] &= 4.8 \times 10^6 \text{ metric tons of } CO_2 \end{align}$$
Notice that the formula weights (FW) of C3H8 and CO2 are coincidentally the same.
-
Consider the reaction:
$KClO_{3 \; (aq)} \longrightarrow KCl_{(aq)} + O_{2 \; (g)}$
- Balance the reaction. What type of reaction is this?
- If 19.5 g of oxygen gas (O2) are formed by this reaction, calculate the amount of KClO3 that must have been present. Assume that the reaction goes to completion. (By the way, it is pretty rare for a reaction to actually go to completion.)
Solution
The balanced equation (a decomposition) is:
$$2 \, KClO_3 \longrightarrow 2 \, KCl + 3 \, O_2$$
The stoichiometry is
$$28 \; \cancel{g \; O_2} \left( \frac{1 \; \cancel{mol \; O_2}}{34 \; \cancel{g \; O_2}} \right) \left( \frac{2 \; \cancel{mol \; KClO_3}}{3 \; \cancel{mol \; O_2}} \right) \left( \frac{122.55 \; g \; KClO_3}{2 \; \cancel{mol \; KClO_3}} \right) = 49.8 \; g \; Cl_2$$
-
Consider the reaction
$K_{(s)} + Cl_{2 \; (ag)} \longrightarrow KCl_{(s)}$
- Balance the reaction. What type of reaction is this?
- If 1.5 g of potassium (K) reacts with 194 g of chlorine gas (Cl2), how much KCl will be formed?
- If 28 g of KCl were formed in this reaction, how much Cl2 (in grams) must have reacted?
Solution
This is a synthesis reaction:
$$2 \, K + Cl_2 \longrightarrow 2 \, KCl$$
Limiting reactant: In this reaction we need twice as many moles of K as Cl2 and we don't have nearly that many (see calculations below).
$$ \begin{align} 1.5 \; \cancel{g \; K} \left( \frac{1 \; mol \; K}{39.1 \; \cancel{g \; K}} \right) = 0.038 \; mol \; K \\[5pt] 194 \; \cancel{g \; Cl_2} \left( \frac{1 \; mol \; Cl_2}{70.9 \; \cancel{g \; Cl_2}} \right) = 2.73 \; mol \; Cl_2 \end{align}$$
So K is the limiting reactant. Now we'll base all further work on the 0.038 moles of K.
$$\text{(b)} \; \; 0.038 \; mol \; K \left( \frac{2 \; \cancel{mol \; KCl}}{2 \; \cancel{mol \; K}} \right) \left( \frac{74.55 \; \cancel{g \; KCl}}{1 \; \cancel{mol \; KCl}} \right) = 2.83 \; g \; KCl$$
$$\text{(c)} \; \; 28 \; \cancel{g \; KCl} \left( \frac{1 \; \cancel{mol \; KCl}}{74.55 \; \cancel{g \; KCl}} \right) \left( \frac{1 \; \cancel{mol \; Cl_2}}{2 \; \cancel{mol \; KCl}} \right) \left( \frac{70.9 \; g \; Cl_2}{1 \; \cancel{mol \; Cl_2}} \right) = 13.3 \; g \; Cl_2$$
-
Consider the reaction:
$Cu_{(s)} + AgNO_{3 \; (aq)} \longrightarrow Cu(NO_3)_{2 \; (aq)} + Ag_{(s)}$
- Balance the reaction. What type of reaction is this?
- How many grams of copper (Cu) would be required, assuming plenty of AgNO3 to react with, to form 2.8 g of silver metal (Ag)?
- If 1.0 g of AgNO3 was reacted with 92.0 g of Cu metal (copper), how much Cu(NO3)2 would be formed in this reaction?
Solution
$Cu + 2 \, AgNO_3 \longrightarrow Cu(NO_3)_2 + 2 \, Ag$ (single displacement)
$$\text{(b)} \; 2.8 \; \cancel{g \; Ag} \left( \frac{1 \; \cancel{mol \; Ag}}{107.9 \; \cancel{g \; Ag}} \right) \left( \frac{1 \; \cancel{mol \; Cu}}{2 \; \cancel{mol \; Ag}} \right) \left( \frac{63.54 \; g \; Cu}{1 \; \cancel{mol \; Cu}} \right) = 0.82 \; g \; Cu$$
Now find the limiting reactant:
$$ \begin{align} 1.0 \; \cancel{g \; AgNO_3} \left( \frac{1 \; mol \; AgNO_3}{169.9 \; \cancel{g \; AgNO_3}} \right) &= 0.0059 \; mol \; AgNO_3 \\[5pt] 92 \; \cancel{g \; Cl_2} \left( \frac{1 \; mol \; Cu}{63.54 \; \cancel{g \; Cu}} \right) &= 1.45 \; mol \; Cu \end{align}$$
In this reaction we need twice as many moles of AgNO3 as Cu and we don't have nearly that many. Therefore AgNO3 is the limiting reactant. Now base all further work on 0.0059 moles of AgNO3.
$$\text{(c)} \; 0.0059 \; \cancel{mol \; AgNO_3} \left( \frac{1 \; \cancel{mol \; Cu(NO_3)_2}}{2 \; \cancel{mol \; AgNO_3}} \right) \left( \frac{187.65 \; g \; Cu(NO_3)_2}{1 \; \cancel{mol \; Cu(NO_3)_2}} \right) = 0.55 \; g \; Cu(NO_3)_2$$
-
Methyl mercaptan (CH4S or CH3SH) is one of the stinky (rotten egg smell) gases added to household natural gas so that humans can detect leaks before being asphyxiated by them. The combustion of methyl mercaptan produces sulfur dioxide:
$CH_4S + 3 \, O_2 \longrightarrow CO_2 + 2 \, H_2O + SO_2$
- How many grams of sulfur dioxide (SO2) are formed from complete combustion of 5 g of CH4S ?
- How many grams of SO2 are formed when 5 g of CH4S react with 2.0 g of O2 ?
Solution
$CH_4S + 3 \; O_2 \longrightarrow CO_2 + 2 \, H_2O + SO_2$ (combusion of a C,H,S compound)
$$\text{(a)} \; 5 \; \cancel{g \; CH_4S} \left( \frac{1 \; \cancel{mol \; CH_4S}}{48 \; \cancel{g \; CH_4S}} \right) \left( \frac{1 \; \cancel{mol \; SO_2}}{1 \; \cancel{mol \; CH_4S}} \right) \left( \frac{64 \; g \; SO_2}{1 \; \cancel{mol \; SO_2}} \right) = 6.67 \; g \; SO_2$$
(b) Here we need to find the limiting reactants:
$$ \begin{align} 5.0 \; \cancel{g \; CH_4S} \left( \frac{1 \; mol \; CH_4S}{48 \; \cancel{g \; CH_4S}} \right) = 0.1042 \; mol \; CH_4S \\[5pt] 2.0 \; \cancel{g \; O_2} \left( \frac{1 \; mol \; Cu}{32 \; \cancel{g \; O_2}} \right) = .0625 \; mol \; O_2 \end{align}$$
In this reaction we need three times as many moles of O2 as CH4S, and we don't have nearly that much, therefore O2 is the limiting reactant. Now base all further work on 0.0625 moles of O2.
$$0.0625 \; \cancel{mol \; O_2} \left( \frac{1 \; \cancel{mol \; SO_2}}{3 \; \cancel{mol \; O_2}} \right) \left( \frac{64 \; g \; SO_2}{1 \; \cancel{mol \; SO_2}} \right) = 1.33 \; g \; SO_2$$
-
Consider the reaction:
$Au_2S_{3 \; (aq)} + H_{2 \; (g)} \longrightarrow H_2S_{(g)} + Au_{(s)}$
If 500.2 g of Au2S3 (gold sulfide) and 5.67 g of H2 react, calculate the amount (in grams) of each of the products.
Solution
Balanced reaction: $Au_2S_3 + 3 \, H_2 \longrightarrow 3 \, H_2S + 2 \, Au$
First determine the limiting reactant:
$$ \begin{align} 500.2 \; \cancel{g \; Au_2S_3} \left( \frac{1 \; mol \; Au_2S_3}{426 \; \cancel{g \; Au_2S_3}} \right) = 1.174 \; mol \; Au_2S_3 \\[5pt] 5.67 \; \cancel{mol \; H_2} \left( \frac{1 \; mol \; H_2}{2 \; \cancel{g \; H_2}} \right) = 2.835 \; mol \; H_2 \end{align}$$
In this reaction we need three times as many moles of H2 as Au2S3, and we don't have quite that many, so H2 is the limiting reactant. Now base all further calculations on 2.835 moles of H2
$$ \begin{align} 2.835 \; \cancel{mol \; H_2} \left( \frac{3 \; \cancel{mol \; H_2S}}{3 \; \cancel{mol \; H_2}} \right) \left( \frac{34.1 \; g \; H_2S}{1 \; \cancel{mol \; H_2S}} \right) = 96.7 \; g \; H_2S \\[5pt] 2.835 \; \cancel{mol \; H_2} \left( \frac{2 \; \cancel{mol \; Au}}{3 \; \cancel{mol \; H_2}} \right) \left( \frac{197 \; g \; Au}{1 \; \cancel{mol \; Au}} \right) = 372 \; g \; Au \end{align}$$
-
Consider the reaction:
$Mg_3N_2 + 6 \, H_2O_{(l)} \longrightarrow 3 \, Mg(OH)_{2 \; (aq)} + 2 \, NH_{3 \; (aq)}$
If 58.1 g of Mg3N2 and 20.4 g of H2O react, calculate the masses of each of the products.
Solution
Balanced: $Mg_3N_2 + 6 \, H_2O \longrightarrow 3 \, Mg(OH)_2 + 2 \, NH_3$
Find the limiting reactant:
$$ \begin{align} 51.8 \; \cancel{g \; Mg_3N_2} \left( \frac{1 \; mol \; Mg_3N_2}{101 \; \cancel{g \; Mg_3N_2}} \right) = 0.513 \; mol \; Mg_3N_2 \\[5pt] 20.4 \; \cancel{g \; H_2O} \left( \frac{1 \; mol \; H_2O}{2 \; \cancel{g \; H_2O}} \right) = 1.133 \; mol \; H_2O \end{align}$$
In this reaction we need six times as many moles of water as Mg3N2, and we have about half that much, so H2O is the limiting reactant. Now base all further calculations on 1.133 moles of H2O.
$$ \begin{align} 1.133 \; \cancel{mol \; H_2O} \left( \frac{3 \; \cancel{mol \; Mg(OH)_2}}{6 \; \cancel{mol \; H_2O}} \right) \left( \frac{58.3 \; g \; Mg(OH)_2}{1 \; \cancel{mol \; Mg(OH)_2}} \right) &= 33.0 \; g \; Mg(OH)_2 \\[5pt] 1.133 \; \cancel{mol \; H_2O} \left( \frac{2 \; \cancel{mol \; NH_3}}{6 \; \cancel{mol \; H_2O}} \right) \left( \frac{17 \; g \; NH_3}{1 \; \cancel{mol \; NH_3}} \right) &= 6.42 \; g \; NH_3 \end{align}$$
Video examples
1. Cu(s) + AgNO3 (aq) → Cu(NO3)2 (aq) + Ag(s)
How many grams of copper metal (assuming excess AgNO3) would it take to form 2.8 g of silver metal, assuming that the reaction proceeded to completion? When you see language like "excess AgNO3" you can assume that this is not a limiting reactant problem.
Minutes of your life: 2:59
2. KClO3 (aq) → KCl (aq) + O2 (g)
If 19.5 g of oxygen (O2) are formed by this reaction, calculate the amount of KClO3 that must have been initially present. Assume that the reaction goes to completion.
Minutes of your life: 2:20
3. A limiting reactant problem
8 g of gold (Au) react with 1.1. liters of Cl2 gas at 2 atm. pressure (T = 250˚C) to produce gold (III) chloride. How much AuCl3 can possibly be produced by this reaction?
In this problem, you'll have to write a balanced reaction first. The number of moles of Cl2 is determined using the ideal gas law: n = PV/RT. Notice that this is a limiting reactant problem because we have fixed amounts of all reactants.
Minutes of your life: 5:18
4. Au2S3 (aq) + H2 (g) → H2S (g) + Au (s)
If 500.2 g of Au2S3 and 5.67 g of H2 react, calculate the amount (in grams) of gold metal that will be formed. Notice that this is a limiting-reactant problem: We know the amounts of both reactants,and it's up to us to determine the maximum amount of product that can be obtained from that mixture.
Minutes of your life: 5:38

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.